Temperature is a way of talking about, measuring, and comparing the thermal energy of objects.
Please login or register to read the rest of this content.
One of the most remarkable images of our planet has always been how dynamic the atmosphere is a photo of the Earth taken from space usually shows swirling masses of white wispy clouds, circling and moving constantly. So what are these graceful puffs that can both frustrate astronomers and excite photographers simultaneously?
Clouds are frozen ice crystals or white liquid water that you can see with your eyes. Scientists who study clouds go into a field of science called nephology, which is a specialized area of meteorology. Clouds don’t have to be made up of water – they can be any visible puff and can have all three states of matter (solid, liquid, and gas) existing within the cloud formation. For example, Jupiter has two cloud decks: the upper are water clouds, and the lower deck are ammonia clouds.
We’re going to learn how to build a weather instrument that will record whether (weather?) the day was sunny or cloudy using a very sensitive piece of paper. Are you ready?
Also known as an udometer or pluviometer or ombrometer, or just plan old ‘rain cup’, this device will let you know how much water came down from the skies. Folks in India used bowls to record rainfall and used to estimate how many crops they would grow and thus how much tax to collect!
These devices reports in “millimeters of rain” or “”centimeters of rain” or even inches of rain”. Sometimes a weather station will collect the rain and send in a sample for testing levels of pollutants.
While collecting rain may seem simple and straightforward, it does have its challenges! Imagine trying to collect rainfall in high wind areas, like during a hurricane. There are other problems, like trying to detect tiny amounts of rainfall, which either stick to the side of the container or evaporate before they can be read on the instrument. And what happens if it rains and then the temperature drops below freezing, before you’ve had a chance to read your gauge? Rain gauges can also get clogged by snow, leaves, and bugs, not to mention used as a water source for birds.
So what’s a scientist to do?
Press onward, like all great scientists! And invent a type of rain gauge that will work for your area. We’re going to make a standard cylinder-type rain gauge, but I am sure you can figure out how to modify it into a weighing precipitation type (where you weigh the amount in the bottle instead of reading a scale on the side), or a tipping bucket type (where a funnel channels the rain to a see-saw that tips when it gets full with a set amount of water) , or even a buried-pit bucket (to keep the animals out).
Please login or register to read the rest of this content.
First invented in the 1600s, thermometers measure temperature using a sensor (the bulb tip) and a scale. Temperature is a way of talking about, measuring, and comparing the thermal energy of objects. We use three different kinds of scales to measure temperature. Fahrenheit, Celsius, and Kelvin. (The fourth, Rankine, which is the absolute scale for Fahrenheit, is the one you’ll learn about in college.)
Mr. Fahrenheit, way back when (18th century) created a scale using a mercury thermometer to measure temperature. He marked 0° as the temperature ice melts in a tub of salt. (Ice melts at lower temperatures when it sits in salt. This is why we salt our driveways to get rid of ice). To standardize the higher point of his scale, he used the body temperature of his wife, 96°.
As you can tell, this wasn’t the most precise or useful measuring device. I can just imagine Mr. Fahrenheit, “Hmmm, something cold…something cold. I got it! Ice in salt. Good, okay there’s zero, excellent. Now, for something hot. Ummm, my wife! She always feels warm. Perfect, 96°. ” I hope he never tried to make a thermometer when she had a fever.
Just kidding, I’m sure he was very precise and careful, but it does seem kind of weird. Over time, the scale was made more precise and today body temperature is usually around 98.6°F.
Later, (still 18th century) Mr. Celsius came along and created his scale. He decided that he was going to use water as his standard. He chose the temperature that water freezes at as his 0° mark. He chose the temperature that water boils at as his 100° mark. From there, he put in 100 evenly spaced lines and a thermometer was born.
Last but not least Mr. Kelvin came along and wanted to create another scale. He said, I want my zero to be ZERO! So he chose absolute zero to be the zero on his scale.
Absolute zero is the theoretical temperature where molecules and atoms stop moving. They do not vibrate, jiggle or anything at absolute zero. In Celsius, absolute zero is -273 ° C. In Fahrenheit, absolute zero is -459°F (or 0°R). It doesn’t get colder than that!
As you can see, creating the temperature scales was really rather arbitrary:
“I think 0° is when water freezes with salt.”
“I think it’s just when water freezes.”
“Oh, yea, well I think it’s when atoms stop!”
Many of our measuring systems started rather arbitrarily and then, due to standardization over time, became the systems we use today. So that’s how temperature is measured, but what is temperature measuring?
Temperature is measuring thermal energy which is how fast the molecules in something are vibrating and moving. The higher the temperature something has, the faster the molecules are moving. Water at 34°F has molecules moving much more slowly than water at 150°F. Temperature is really a molecular speedometer.
Let’s make a quick thermometer so you can see how a thermometer actually works:
Hygrometers measure how much water is in the air, called humidity. If it's raining, it's 100% humidity. Deserts and arid climates have low humidity and dry skin. Humidity is very hard to measure accurately, but scientists have figured out ways to measure how much moisture is absorbed by measuring the change in temperature (as with a sling psychrometer), pressure, or change in electrical resistance (most common).
The dewpoint is the temperature when moist air hits the water vapor saturation point. If the temperature goes below this point, the water in the air will condense and you have fog. Pilots look for temperature and dewpoint in their weather reports to tell them if the airport is clear, or if it''s going to be 'socked in'. If the temperature stays above the dewpoint, then the airport will be clear enough to land by sight. However, if the temperature falls below the dewpoint, then they need to land by instruments, and this takes preparation ahead of time.
A sling psychrometer uses two thermometers (image above), side by side. By keeping one thermometer wet and the other dry, you can figure out the humidity using a humidity chart. Such as the one on page two of this document. The psychrometer works because it measures wet-bulb and dry-bulb temperatures by slinging the thermometers around your head. While this sounds like an odd thing to do, there's a little sock on the bottom end of one of the thermometers which gets dipped in water. When air flows over the wet sock, it measures the evaporation temperature, which is lower than the ambient temperature, measured by the dry thermometer.
Scientists use the difference between these two to figure out the relative humidity. For example, when there's no difference between the two, it's raining (which is 100% humidity). But when there's a 9oC temperature difference between wet and dry bulb, the relative humidity is 44%. If there's 18oC difference, then it's only 5% humidity.
You can even make your own by taping two identical thermometers to cardboard, leaving the ends exposed to the air. Wrap a wet piece of cloth or tissue around the end of one and use a fan to blow across both to see the temperature difference!
One of the most precise are chilled mirror dewpoint hygrometers, which uses a chilled mirror to detect condensation on the mirror's surface. The mirror's temperature is controlled to match the evaporation and condensation points of the water, and scientists use this temperature to figure out the humidity.
We're going to make a very simple hygrometer so you get the hand of how humidity can change daily. Be sure to check this instrument right before it rains. This is a good instrument to read once a day and log it in your weather data book.

A barometer uses either a gas (like air) or a liquid (like water or mercury) to measure pressure of the atmosphere. Scientists use barometers a lot when they predict the weather, because it’s usually a very accurate way to predict quick changes in the weather.
Barometers have been around for centuries – the first one was in the 1640s!
At any given momen, you can tell how high you are above sea level by measure the pressure of the air. If you measure the pressure at sea level using a barometer, and then go up a thousand feet in an airplane, it will always indicate exactly 3.6 kPa lower than it did at sea level.
Scientists measure pressure in “kPa” which stands for “kilo-Pascals”. The standard pressure is 101.3 kPa at sea level, and 97.7 kPa 1,000 feet above sea level. In fact, every thousand feet you go up, pressure decreases by 4%. In airplanes, pilots use this fact to tell how high they are. For 2,000 feet, the standard pressure will be 94.2 kPa. However, if you’re in a low front, the sea level pressure reading might be 99.8 kPa, but 1000 feet up it will always read 3.6 kPa lower, or 96.2 kPa.
Most weather stations have anemometers to measure wind speed or wind pressure. The kind of anemometer we’re going to make is the same one invented back in 1846 that measures wind speed. Most anemometers use three cups, which is not only more accurate but also responds to wind gusts more quickly than a four-cup model.
Some anemometers also have an aerovane attached, which enables scientists to get both speed and direction information. It looks like an airplane without wings – with a propeller at the front and a vane at the back.
Other amemometers don’t have any moving parts – instead they measure the resistance of a very short, thin piece of tungsten wire. (Resistance is how much a substance resists the flow of electrical current. Copper has a low electrical resistance, whereas rubber has a very high resistance.) Resistance changes with the material’s temperature, so the tungsten wire is heated and placed in the airflow. The wind flowing over the wire cools it down and increases the resistance of the wire, and scientists can figure out the wind speed.
Being able to predict tomorrow’s weather is one of the most challenging and frequently requested bits of information to provide. Do you need a coat tomorrow? Will soccer practice be canceled? Will the crops freeze tonight?
Scientists use different instruments to record the current weather conditions, like temperature, barometric pressure, wind speed, humidity, etc. The real work comes in when they spend time looking over their data over days, months, even years and search for patterns.
But where does the weather station get its weather from?
One of the greatest leaps in meteorology was using numbers to predict the flow of the atmosphere. The math equations needed for these (using fluid dynamics and thermodynamics) are enough to make even a graduate student quiver with fear. Even today’s most powerful computers cannot solve these complex equations! The best they can do is make a guess at the solution and then adjust it until it fits well enough in a given range. How do the computers know what to guess?
Several weather stations around the world work together to report the current weather every hour. These stations can be land-based, mounted on buoys in the ocean, or launched on radiosondes and report back to a home station as they rise through the different layers of the atmosphere. Pilots will also give weather reports en route to their destination, which get recorded and added to the database of weather knowledge.
We’re going to build our own homemade weather station and start keeping track of weather right in your own home town. By keeping a written record (even if it’s just pen marks on the wall), you’ll be able to see how the weather changes and even predict what it will do, once you get the hang of the pattern in your local area. For example, if you live in Florida, what happens to the pressure before the daily afternoon thunderstorm? Or if you live in the deserts of Arizona, what does a sudden increase in humidity tell you?
Please login or register to read the rest of this content.
This is a recording of a recent live teleclass I did with thousands of kids from all over the world. I’ve included it here so you can participate and learn, too!
You’ll discover how to boil water at room temperature, heat up ice to freeze it, make a fire water balloon, and build a real working steam boat as you learn about heat energy. You’ll also learn about thermal energy, heat capacity, and the laws of thermodynamics.
Materials:
The energy of sunlight powers our biosphere (air, water, land, and life on the earth’s surface). About 50 percent of the solar energy striking the earth is converted to heat that warms our planet and drives the winds. About 30 percent of the solar energy is reflected directly back into space. The water cycle (evaporation of water followed by rain or snow) is powered by about 20 percent of the solar energy.
Some of the sunlight that reaches the earth is used by plants in photosynthesis. Plants containing chlorophyll use photosynthesis to change sunlight to energy. Since these green plants form the base of the food chain, all plants and animals depend on solar energy for their survival.
When the sun is overhead, about 1,000 watts of solar power strike 1 square meter (10.8 square feet) of the earth’s surface. Using solar cells, this solar energy can be converted to electricity. However, because sunlight cannot be converted completely to electricity, it takes at least a square meter of area to gather enough sunlight to run a 100-watt light bulb.
Solar energy is still more expensive than other methods of generating electricity. However, the cost of solar electricity has greatly decreased since the first solar cells were developed in 1954.
It has been proposed that panels of solar cells on satellites in orbit above the earth could convert solar energy to electricity twenty-four hours a day. These huge solar power satellites could convert electrical energy to microwaves and then beam these microwaves to Earth. At the earth’s surface, tremendous fields covered with antennas could convert the microwave energy back to electricity.
It would take thousands of astronauts many years to build such a complicated system. However, there are many practical uses of solar energy in use today. These uses include heating water, heating and cooling buildings, producing electricity from solar cells, and using rain and snow from the water cycle to power electrical generators at dams.
In the following experiments, you will examine the use of solar energy in heating water, .cooking foods, concentrating sunlight, and producing electricity.
Please login or register to read the rest of this content.
The United States has large reserves of coal, natural gas, and crude oil which is used to make gasoline. However, the United States uses the energy of millions of barrels of crude oil every day, and it must import about half its crude oil from other countries.
Burning fossil fuels (oil, coal, gasoline, and natural gas) produces carbon dioxide gas. Carbon dioxide is one of the main greenhouse gases that may contribute to global warming. In addition, burning coal and gasoline can produce pollution molecules that contribute to smog and acid rain.
Using renewable energy-such as solar, wind, water, biomass, and geothermal-could help reduce pollution, prevent global warming, and decrease acid rain. Nuclear energy also has these advantages, but it requires storing radioactive wastes generated by nuclear power plants. Currently, renewable energy produces only a small part of the energy needs of the
United States. However, as technology improves, renewable energy should become less expensive and more common.
Hydropower (water power) is the least expensive way to produce I electricity. The sun causes water to evaporate. The evaporated water falls to the earth as rain or snow and fills lakes. Hydropower uses water stored in lakes behind dams. As water flows through a dam, the falling water turns turbines that run generators to produce electricity.
Currently, geothermal energy (heat inside the earth), biomass (energy from plants), solar energy (light from concentrated sunlight), and wind are being used to generate electricity. For example, in California there are more than sixteen thousand (16,000) wind turbines that generate enough power to supply a city the size of San Francisco with electricity.
In addition to producing more energy, we can also help meet our energy needs through conservation. Conservation means using less energy and using it more efficiently.
In the following experiments, you will use wind to do work, examine how batteries can store energy, and see how insulation can save energy.
Please login or register to read the rest of this content.
Temperature is a measure of the average hotness of an object. The hotter an object, the higher its temperature. As the temperature is raised, the atoms and molecules in an object move faster. The molecules in hot water move faster than the molecules in cold water. Remember that the heat energy stored in an object depends on both the temperature and the amount of the substance. A smaller amount of water will have less heat energy than a larger amount of water at the same temperature.
Increasing the temperature of a large body of water is one way to store heat energy for later use. A large container filled with salt water, called brine, may be used to absorb heat energy during the day when it is warm. This energy will be held in the salt water until the night when it is cooler. This stored heat energy can be released at night to warm a house or building. This is one way to store the sun’s heat energy until it is needed.
Solar ponds are used to store energy from the sun. Temperatures close to 100°C (212°F) have been achieved in solar ponds. Solar ponds contain a layer of fresh water above a layer of salt water. Because the salt water is heavier, it remains at the bottom of the pond-even as it gets quite hot. A black plastic bottom helps absorb solar energy from sunlight. The water on top serves to insulate and trap the heat in the pond.
In a fresh water pond, as the water on the bottom is heated from sunlight, the hot water becomes lighter and rises to the top of the pond. This convection or movement of hot water to the top tends to carry away excess heat. However, in a salt water pond, there is no convection so heat is trapped. In Israel a series of salt water, solar ponds were developed around the Dead Sea. The heat stored in these solar ponds has been used to run turbines and generate electricity.
Please login or register to read the rest of this content.
Without the sun, there would be no life on Earth. The sun warms the earth, generates wind, and carries water into the air to produce rain and snow. The energy of the sun provides sunlight for all the plant life on our planet, and through plants provides energy for all animals.
The sun is like a giant furnace in which hydrogen nuclei (atoms without electrons) are constantly smashed together to form helium nuclei. This process is called nuclear fusion. In this process, 3.6 billion kilograms (8 billion pounds) of matter are converted to pure energy every second. The temperature in the sun exceeds 15 million degrees.
Nuclear fusion is one kind of energy. Other forms of energy include: mechanical energy, heat, electrical energy, chemical energy, and light. Mechanical energy is the energy of organized motion, such as a turning wheel. Heat is the energy of random motion, such as a cup of hot water. Electrical energy is the energy of moving charged particles or electrons, such as a current in a wire. Chemical energy is the energy stored in bonds that hold atoms together. Light is any form of electromagnetic waves, such as X rays, microwaves, radio waves, ultraviolet light, or visible light.
Energy can be converted from one from to another. For example, the nuclear energy of the sun is converted to light, which goes through space to the earth. Solar collectors of mirrors can be used to focus some of that light to heat water to steam. This steam can be used to turn a turbine, which can power a generator to produce electricity.
Most of our energy needs are met by burning fossil fuels such as coal, oil, gasoline, and natural gas. The chemical energy stored in these substances is released by burning these fuels. When fossil fuels burn, they combine with oxygen in the air and produce heat and light.
Fossil fuels are not renewable. When they are used up, they are gone forever. However, renewable energy sources such as wind, sun, geothermal, biomass and water power are renewable. They can be used over and over to generate the energy to run our society.
Tremendous amounts of renewable energy are available. For example, the solar energy that falls on just the road surfaces in the United States is equal to the entire energy needs of the country. Although there are sufficient amounts of renewable energy, we must improve our methods of collecting, concentrating, and converting renewable energy into useful forms.
In the following experiments, you will learn something about the amount of energy the sun produces at the earth’s surface and how heat energy can be stored.
Please login or register to read the rest of this content.
 Fossil fuels, which include petroleum, natural gas, and coal, supply nearly 90 percent of the energy needs of the United States and other industrialized nations. Because of their high demand, these nonrenewable energy resources are rapidly being consumed. Coal supplies are expected to last about a thousand years.
Fossil fuels, which include petroleum, natural gas, and coal, supply nearly 90 percent of the energy needs of the United States and other industrialized nations. Because of their high demand, these nonrenewable energy resources are rapidly being consumed. Coal supplies are expected to last about a thousand years.
We must find other sources of energy to meet the increasing fuel demands of modern society. Important alternate sources of energy include: solar, wind, biomass, hydroelectric, geothermal, nuclear, and tidal energy.
One of the benefits of using alternate sources of energy is that many of them are “clean.” This means that they do not cause pollution. Also, many alternative energy sources are renewable energy sources. They are replaced naturally-such as plant life-or are readily available – such as the sun and wind. In addition, the use of renewable forms of energy will allow us to stretch out our current supply of fossil fuels so they will last longer.
In this chapter you will learn how biomass, or organic matter, can be an important energy source. Plants are the most important biomass energy source. Plant material can be burned directly-as with wood-or it can be converted into a fuel by other means. In the experiments that follow you will explore: how water can be heated by composting grass, how a peanut burns, and how corn syrup can be made into ethyl alcohol.
Please login or register to read the rest of this content.
A peanut is not a nut, but actually a seed. In addition to containing protein, a peanut is rich in fats and carbohydrates. Fats and carbohydrates are the major sources of energy for plants and animals.
The energy contained in the peanut actually came from the sun. Green plants absorb solar energy and use it in photosynthesis. During photosynthesis, carbon dioxide and water are combined to make glucose. Glucose is a simple sugar that is a type of carbohydrate. Oxygen gas is also made during photosynthesis.
The glucose made during photosynthesis is used by plants to make other important chemical substances needed for living and growing. Some of the chemical substances made from glucose include fats, carbohydrates (such as various sugars, starch, and cellulose), and proteins.
Photosynthesis is the way in which green plants make their food, and ultimately, all the food available on earth. All animals and nongreen plants (such as fungi and bacteria) depend on the stored energy of green plants to live. Photosynthesis is the most important way animals obtain energy from the sun.
Oil squeezed from nuts and seeds is a potential source of fuel. In some parts of the world, oil squeezed from seeds-particularly sunflower seeds-is burned as a motor fuel in some farm equipment. In the United States, some people have modified diesel cars and trucks to run on vegetable oils.
Fuels from vegetable oils are particularly attractive because, unlike fossil fuels, these fuels are renewable. They come from plants that can be grown in a reasonable amount of time.
Please login or register to read the rest of this content.
Yeast is a simple living organism that can break down sugars into ethyl alcohol (ethanol) and carbon dioxide. The process by which yeast breaks down sugars into ethyl alcohol and carbon dioxide is called fermentation.
The tiny gas bubbles rising in the liquid mixture in the bottle are carbon dioxide gas bubbles that are made during the fermentation. The balloon on the bottle expands and becomes inflated because it traps the carbon dioxide gas being produced.
The ethyl alcohol that is made during fermentation stays in the liquid mixture. When fermentation is finished, the liquid mixture usually contains about 13 percent ethyl alcohol. The rest of the liquid is mostly water.
The ethyl alcohol can be concentrated by a process called distillation. During distillation, the liquid fermentation mixture is heated to change the ethyl alcohol and some of the water into a vapor. The vapor is then cooled to change it back into a liquid. This distilled liquid contains 95 percent ethyl alcohol and 5 percent water. The remaining water can be removed by special distillation methods to give pure ethyl alcohol.
In some areas of the United States, ethyl alcohol is blended with gasoline to make a motor fuel known as gasohol. About 8 percent of the gasoline sold in the United States is gasohol.
Gasohol burns more cleanly than pure gasoline. This results in fewer pollutants being released into the air. The use of gasohol as a motor fuel is particularly important in cities that have a lot of smog.
Corn syrup is a mixture of simple and complex sugars and water. It is made by breaking down the starch in corn into sugars. The process is called digestion. In this experiment you changed the sugars in corn syrup using yeast. Much of the ethyl alcohol used to prepare gasohol is made by fermenting corn and corn sugar.
Over one billion gallons of ethyl alcohol are made each year by fermentation of sugars from grains such as corn. Ethyl alcohol is a renewable energy source when it is made by fermenting grains such as corn. This is because the grains, such as corn, are easily grown.
 The atoms in a solid, as we mentioned before, are usually held close to one another and tightly together. Imagine a bunch of folks all stuck to one another with glue. Each person can wiggle and jiggle but they can’t really move anywhere.
The atoms in a solid, as we mentioned before, are usually held close to one another and tightly together. Imagine a bunch of folks all stuck to one another with glue. Each person can wiggle and jiggle but they can’t really move anywhere.
Atoms in a solid are the same way. Each atom can wiggle and jiggle but they are stuck together. In science, we say that the molecules have strong bonds between them. Bonds are a way of describing how atoms and molecules are stuck together.
There’s nothing physical that actually holds them together (like a tiny rope or something). Like the Earth and Moon are stuck together by gravity forces, atoms and molecules are held together by nuclear and electromagnetic forces. Since the atoms and molecules come so close together they will often form crystals.
Try this experiment and then we will talk more about this:
Please login or register to read the rest of this content.
Crystals are formed when atoms line up in patterns and solidify. There are crystals everywhere — in the form of salt, sugar, sand, diamonds, quartz, and many more!
To make crystals, you need to make a very special kind of solution called a supersaturated solid solution. Here’s what that means: if you add salt by the spoonful to a cup of water, you’ll reach a point where the salt doesn’t disappear (dissolve) anymore and forms a lump at the bottom of the glass.
The point at which it begins to form a lump is just past the point of saturation. If you heat up the saltwater, the lump disappears. You can now add more and more salt, until it can’t take any more (you’ll see another lump starting to form at the bottom). This is now a supersaturated solid solution. Mix in a bit of water to make the lump disappear. Your solution is ready for making crystals. But how?
Popcorn rocks are different than regular dolomite samples because they have a lot more magnesium inside. This was first discovered by a geology professor in the 1980s who was dissolving the limestone around fossils he was studying in his rock samples. When he placed samples of this type in the acid to dissolve, it didn’t dissolve but instead grew new crystals!
This is a recording of a recent live teleclass I did with thousands of kids from all over the world. I've included it here so you can participate and learn, too! Learn about the world of rocks, crystals, gems, fossils, and minerals by moving beyond just looking at pretty stones and really being able to identify, test, and classify samples and specimens you come across using techniques that real field experts use. While most people might think of a rock as being fun to climb or toss into a pond, you will now be able to see the special meaning behind the naturally occurring material that is made out of minerals by understanding how the minerals are joined together, what their crystalline structure is like, and much more.
Materials:
 If you’ve ever eaten fruits or vegetables (and let’s hope you have), you have benefited from plants as food. Of course, the plants we eat have been highly modified by growers to produce larger and sweeter fruit, or heartier vegetables.
If you’ve ever eaten fruits or vegetables (and let’s hope you have), you have benefited from plants as food. Of course, the plants we eat have been highly modified by growers to produce larger and sweeter fruit, or heartier vegetables.
There are three basic ways to create plants with new, more desirable traits:
Six-foot zucchini? Ten-foot carrots? Are giant veggies just a photography trick, or are they real?
The happy news is that yes, they’re real! Expert horticulturists have accumulated a great wealth of knowledge about different climates and dirt conditions. They must know about the different chemical, physical and biological properties of gardens and do multiples of experiments dozens of plants. We found an incredible horticulturist, John Evans, who has accumulated over 180 first places in both quality and giant vegetable categories, with 18 State and 7 World Records.
According to John Evans: “If you could, imagine what it would be like to dig up a carrot from your garden and not knowing how big it is until the last minute, and then finding out that it’s 19 lbs. Now that’s exciting!”
John has spent many years developing fertilizers, bio-catalysts, and growing techniques to grow 76-lb cabbages (photo shown left), 20-lb carrots, 29-lb kale, 60-lb zucchini, 43-lb beets, 35-lb broccoli and cauliflowers, and 70-lb swiss chard that was over 9 feet tall and took three people to carry it to the trailer!
Here’s a video on growing giant flowers by a passionate community gardening club:
Please login or register to read the rest of this content.
If you’re thinking sunlight, you’re right. Natural light is best for plants for any part of the plant’s life cycle. But what can you offer indoor plants?
In Unit 9 we learned how light contains different colors (wavelengths), and it’s important to understand which wavelengths your indoor plant prefers.
Plants make their food through photosynthesis: the chlorophyll transforms carbon dioxide into food. Three things influence the growth of the plant: the intensity of the light, the time the plant is exposed to light, and the color of the light.
When plants grow in sunlight, they get full intensity and the full spectrum of all wavelengths. However, plants only really use the red and blue wavelengths. Blue light helps the leaves and stems grow (which means more area for photosynthesis) and seedlings start, so fluorescent lights are a good choice, since they are high in blue wavelengths.
 Broccoli, like all plants, has chlorophyll, making it green. You can really “see” the chlorophyll when you boil broccoli. This is such a simple experiment that you can do this as you prepare dinner tonight with your kids. Make sure you have an extra head of broccoli for this experiment, unless you really like to eat overcooked broccoli.
Broccoli, like all plants, has chlorophyll, making it green. You can really “see” the chlorophyll when you boil broccoli. This is such a simple experiment that you can do this as you prepare dinner tonight with your kids. Make sure you have an extra head of broccoli for this experiment, unless you really like to eat overcooked broccoli.
 Mass and energy are conserved. This means you can’t create or destroy them, but you can change their location or form.
Mass and energy are conserved. This means you can’t create or destroy them, but you can change their location or form.
Most people don’t understand that the E energy term means all the energy transformations, not just the nuclear energy.
The energy could be burning gasoline, fusion reactions (like in the sun), metabolizing your lunch, elastic energy in a stretched rubber band… every kind of energy stored in the mass is what E stands for.
For example, if I were to stretch a rubber band and somehow weigh it in the stretched position, I would find it weighed slightly more than in the unstretched position.
Why? How can this be? I didn’t add any more particles to the system – I simply stretched the rubber band. I added energy to the system, which was stored in the electromagnetic forces inside the rubber band, which add to the mass of the object (albeit very slightly). Read more about this in Unit 7: Lesson 3.
Why do families share similar features like eye and hair color? Why aren’t they exact clones of each other? These questions and many more will be answered as well look into the fascinating world of genetics!
Genetics asks which features are passed on from generation to generation in living things. It also tries to explain how those features are passed on (or not passed on). Which features are stay and leave depend on the genes of the organism and the environment the organism lives in. Genes are the “inheritance factors “described in Mendel’s laws. The genes are passed on from generation to generation and instruct the cell how to make proteins. A genotype refers to the genetic make-up of a trait, while phenotype refers to the physical manifestation of the trait.
We’re going to create a family using genetics!
Insects are not only the most diverse subgroup of arthropods, but with over a million discovered species it is the most diverse group of animals on earth. Although they can’t all be as beautiful as a butterfly, they all play important roles in their ecosystems—just think of where we would be without bees!
Some insects are just too small! Even if we try to carefully pick them up with forceps, they either escape or are crushed. What to do?
Answer: Make an insect aspirator! An insect aspirator is a simple tool scientists use to collect bugs and insects that are too small to be picked up manually. Basically it’s a mini bug vacuum!
Please login or register to read the rest of this content.
The way animals and plants behave is so complicated because it not only depends on climate, water availability, competition for resources, nutrients available, and disease presence but also having the patience and ability to study them close-up.
We’re going to build an eco-system where you’ll farm prey stock for the predators so you’ll be able to view their behavior. You’ll also get a chance to watch both of them feed, hatch, molt, and more! You’ll observe closely the two different organisms and learn all about the way they live, eat, and are eaten.
When you hear “roach” you might not immediately think of something that would make a good pet, but not all roaches are like the cockroaches you might have seen in your house!
Species such as the Orange Spotted Roach (Blaptica dubia) make excellent insect pets: they don’t cost much, they have an interesting life cycle and habits, and they do not require much effort to care for. Their average lifespan is about 18 months and you’ll be able to learn more about their fascinating life cycle (from egg to adult) if you allow them to breed!
This experiment is for advanced students.
ACID!!! The word causes fear to creep in and get our attention.
BASIC!!! The word causes nothing to stir in most of us.
The truth is, a strong acid (pH 0-1) is dangerous, but a strong basic (pH 13-14) is just as dangerous. In this lab, we will get comfortable with the basics of bases and the acidity of acids along with how you can use both and tell the difference between them.
Cobalt chloride (CoCl2) has a dramatic color change when combined with water, making it a great water indicator. A concentrated solution of cobalt chloride is red at room temperature, blue when heated, and pale-to-clear when frozen. The cobalt chloride we’re using is actually cobalt chloride hexahydrate, which means that each CoCl2 molecule also has six water molecules (6H2O) stuck to it.
You can go your whole life without paying any attention to the chemistry behind acids and bases. But you use acids and bases all the time! They are all around you. We identify acids and bases by measuring their pH.
Every liquid has a pH. If you pay particular attention to this lab, you will even be able to identify most acids and bases and understand why they do what they do. Acids range from very strong to very weak. The strongest acids will dissolve steel. The weakest acids are in your drink box. The strongest bases behave similarly. They can burn your skin or you can wash your hands with them.
Acid rain is one aspect of low pH that you can see every day if you look for it. This is a strange name, isn’t it? We get rained on all the time. If people were dissolving, if the rain made their skin smoke and burn, you’d think it would make headlines, wouldn’t you? The truth is acid rain is too weak to harm us except in very rare and localized conditions. But it’s hard on limestone buildings.
Mars is coated with iron oxide, which not only covers the surface but is also present in the rocks made by the volcanoes on Mars.
Today you get to perform a chemistry experiment that investigates the different kinds of rust and shows that given the right conditions, anything containing iron will eventually break down and corrode. When iron rusts, it’s actually going through a chemical reaction: Steel (iron) + Water (oxygen) + Air (oxygen) = Rust
Materials
This experiment is for advanced students.Have you ever taken a gulp of the ocean? Seawater can be extremely salty! There are large quantities of salt dissolved into the water as it rolled across the land and into the sea. Drinking ocean water will actually make you thirstier (think of eating a lot of pretzels). So what can you do if you’re deserted on an island with only your chemistry set?
Let me show you how to take the salt out of water with this easy setup.
This experiment is for advanced students.
Don’t put this in your car….yet. Hydrogen generation, capture, and combustion are big deals right now. The next phase of transportation, and a move away from fossil fuels in not found in electric cars. Electric cars are waiting until hydrogen fuel cell vehicles become practical. It can be done and is being done.
Cars being powered by hydrogen are here, but not on the market yet. Engineers and chemists are always finding new ways to improve the chemical reaction that produces hydrogen and making the vehicles more efficiently use the fuel. Hydrogen fuel is not just easy to make, it is inexpensive, and the “exhaust” is water.
We will generate hydrogen in this lab. We will also see how combustible it is. Just let your imagination wander….just a bit and you will see noiseless cars and trucks zipping along the streets and interstates, carrying people and cargo. The Indianapolis 500 wouldn’t be quite the same, though. “And there they go, roaring, I mean quietly entering turn two…”
Guar gum comes from the guar plant (also called the guaran plan), and people have found a lot of different and interesting uses for it. It's one of the primary substitutes for fat in low-fat and fat-free foods. Cooks like to use guar gum in foods as it has 8 times the thickening power of cornstarch, so much less is needed for the recipe. Ice cream makers use it to keep ice crystals from forming inside the carton. Doctors use it as a laxative for their patients.
When we teach kids how to make slime using guar gum, they call it "fake fat" slime, mostly because it's used in fat-free baking. You can find guar gum in health food stores or order it online. We're going to whip up a batch of slime using this "fake fat". Ready?
Here's what you do:
Please login or register to read the rest of this content.
The glue is a polymer, which is a long chain of molecules all hooked together like tangled noodles. When you mix the two solutions together, the water molecules start linking up the noodles together all along the length of each noodle to get more like a fishnet. Scientists call this a polymetric compound of sodium tetraborate and lactated glue. We call it bouncy putty.
Here’s what you do:
This experiment is for advanced students.
Lewis and Clark did this same experiment when they reached the Oregon coast in 1805. Men from the expedition traveled fifteen miles south of the fort they had built at the mouth of the Columbia River to where Seaside, Oregon now thrives.
In 1805, however, it was just men from the fort and Indians. They built an oven of rocks. For six weeks, they processed 1,400 gallons of seawater, boiling the water off to gain 28 gallons of salt.
 Have you ever tried washing dishes without soap? It doesn’t work well, especially if there’s a lot of grease, fat, or oil on the dish!
Have you ever tried washing dishes without soap? It doesn’t work well, especially if there’s a lot of grease, fat, or oil on the dish!
The oils and fats are slippery and repel water, which makes them a great choice for lubration of bearing and wheels, but lousy for cleaning up after dinner.
So what’s inside soap that makes it clean off the dish? The soap molecule looks a lot like a snake, with a head and a tail. The long tail loves oil (hydrophobic) and the head loves water (hydrophilic). The hydrophilic end dissolves in water and the hydrophobic end wraps itself around fat and oil in the dirty water, cleaning it off your dishes.
Let’s do an experiment that will really make you appreciate soap and fat:
If you’ve ever burped, you know that it’s a lot easier to do after chugging an entire soda. Now why is that?
Soda is loaded with gas bubbles — carbon dioxide (CO2), to be specific. And at standard temperature (68oF) and pressure (14.7 psi), carbon dioxide is a gas. However, if you burped in Antarctica in the wintertime, it would begin to freeze as soon as it left your lips. The freezing temperature of CO2 is -109oF, and Antarctic winters can get down to -140oF. You’ve actually seen this before, as dry ice (frozen burps!).
Carbon dioxide has no liquid state at low pressures (75 psi or lower), so it goes directly from a block of dry ice to a smoky gas (called sublimation). It’s also acidic and will turn cabbage juice indicator from blue to pink. CO2 is colorless and odorless, just like water, but it can make your mouth taste sour and cause your nose to feel as if it’s swarming with wasps if you breathe in too much of it (though we won’t get anywhere near that concentration with our experiments).
The triple point of CO2 (the point at which CO2 would be a solid, a liquid, and a gas all at the same time) is around five times the pressure of the atmosphere (75 psi) and around -70oF. (What would happen if you burped then?)
What sound does a fresh bottle of soda make when you first crack it open? PSSST! What is that sound? It’s the CO2 (carbon dioxide) bubbles escaping. What is the gas you exhale with every breath? Carbon dioxide. Hmmm … it seems as if your soda is already pre-burped. Interesting.
We’ll actually be doing a few different experiments, but they all center around producing burps (carbon dioxide gas). The first experiment is more detective work in finding out where the CO2 is hiding. With the materials we’ve listed (chalk, tile, limestone, marble, washing soda, baking soda, vinegar, lemon juice, etc. …) and a muffin tin, you can mix these together and find the bubbles that form, which are CO2. (Not all will produce a reaction.) You can also try flour, baking powder, powdered sugar, and cornstarch in place of the baking soda. Try these substitutes for the vinegar: water, lemon juice, orange juice, and oil.
Materials:
This experiment is for advanced students. This lab builds on concepts from the previous carbon dioxide lab.
Limewater….carbon dioxide…indicators. We don’t know too much about these things. Sure, we know a little. Carbon dioxide is exhaled by us and plants need it to grow. Burning fossil fuels produces carbon dioxide.
Indicators…something we observe that confirms to us that something specific is happening. Lime water turns cloudy and forms a precipitate in the presence of carbon dioxide. Blue litmus paper turns red in the presence of an acid. The dog barking at the door and dancing around indicates that you better let the dog out, and quick, to avoid….a pet spill?
CAUTION!! Be careful with this!! This experiment uses a knife AND a microwave, so you’re playing with things that slice and gets things hot. If you’re not careful you could cut yourself or burn yourself. Please use care!
We’re going to create the fourth state of matter in your microwave using food. Note – this is NOT the kind of plasma doctors talk about that’s associated with blood. These are two entirely different things that just happen to have the same name. It’s like the word ‘trunk’, which could be either the storage compartment of a car or an elephant’s nose. Make sense?
Plasma is what happens when you add enough energy (often in the form of raising the temperature) to a gas so that the electrons break free and start zinging around on their own. Since electrons have a negative charge, having a bunch of free-riding electrons causes the gas to become electrically charged. This gives some cool properties to the gas. Anytime you have charged particles (like naked electrons) off on their own, they are referred to by scientists as ions. Hopefully this makes the dry textbook definition make more sense now (“Plasma is an ionized gas.”)
So here’s what you need:
Density is basically how tightly packed atoms are. (Mathematically, density is mass divided by volume.) For example, take a golf ball and a ping pong ball. Both are about the same size or, in other words, take up the same volume.
However, one is much heavier, has more mass, than the other. The golf ball has its atoms much more closely packed together than the ping pong ball and as such the golf ball is denser.
These are quick and easy demonstrations for density that use simple household materials:
Please login or register to read the rest of this content.
Did you know that most people can’t crack an egg with only one hand without whacking it on something? The shell of an egg is quite strong! Try this over a sink and see if you can figure out the secret to cracking an egg in the palm of your hand…(Hint: the answer is below the video – check it out after you’ve tried it first!)
How can you tell if an egg is cooked or raw? Simply spin it on the counter and you’ll get a quick physics lesson in inertia…although you might not know it. A raw egg is all sloshy inside, and will spin slow and wobbly. A cooked egg is all one solid chunk, so it spins quickly. Remember the Chicken and the Clam experiment?
 When you warm up leftovers, have you ever wondered why the microwave heats the food and not the plate? (Well, some plates, anyway.) It has to do with the way microwaves work.
When you warm up leftovers, have you ever wondered why the microwave heats the food and not the plate? (Well, some plates, anyway.) It has to do with the way microwaves work.
Microwaves generate high energy electromagnetic waves that when aimed at water molecules, makes these molecules get super-excited and start bouncing around a lot.
We see this happen when we heat water in a pot on the stove. When you add energy to the pot (by turning on the stove), the water molecules start vibrating and moving around faster and faster the more heat you add. Eventually, when the pot of water boils, the top layer of molecules are so excited they vibrate free and float up as steam.
When you add more energy to the water molecule, either by using your stove top or your nearest microwave, you cause those water molecules to vibrate faster. We detect these faster vibrations by measuring an increase in the temperature of the water molecules (or in the food containing water). Which is why it’s dangerous to heat anything not containing water in your microwave, as there’s nowhere for that energy to go, since the electromagnetic radiation is tuned to excite water molecules.
To explain this to younger kids (who might confuse radio waves with sounds waves) you might try this:
There’s light everywhere, some of which you can see (like rainbows) and others that you can’t see (like the infrared beam coming from your TV remote, or the UV rays from the sun that give you a sunburn). The microwave shoots invisible light beams at your food that are tuned to heat up the water molecule.
The microwave radiation emitted by the microwave oven can also excite other polarized molecules in addition to the water molecule, which is why some plates also get hot. The soap in this experiment below will show you how a bar of Ivory soap contains air, and that air contains water vapor which will get heated by the microwave radiation and expand. Are you ready?
Here’s what you need:
If you soak chicken bones in acetic acid (distilled vinegar), you’ll get rubbery bones that are soft and pliable as the vinegar reacts with the calcium in the bones. This happens with older folks when they lose more calcium than they can replace in their bones, and the bones become brittle and easier to break. Scientists have discovered calcium is replaced more quickly in bodies that exercise and eating calcium rich foods, like green vegetables.
This is actually two experiments in one – here’s what you need to do:
A non-Newtonian fluid is a substance that changes viscosity, such as ketchup. Ever notice how ketchup sticks to the bottom of the bottle one minute and comes sliding out the next?
Think of viscosity as the resistance stuff has to being smeared around. Water is “thin” (low viscosity); honey is “thick” (high viscosity). You are about to make a substance that is both (low and high viscosity), depending on what ratio you mix up. Feel free to mix up a larger batch then indicated in the video – we’ve heard from families that have mixed up an entire kiddie pool of this stuff!
 Can we really make crystals out of soap? You bet! These crystals grow really fast, provided your solution is properly saturated. In only 12 hours, you should have sizable crystals sprouting up.
Can we really make crystals out of soap? You bet! These crystals grow really fast, provided your solution is properly saturated. In only 12 hours, you should have sizable crystals sprouting up.
You can do this experiment with either skewers, string, or pipe cleaners. The advantage of using pipe cleaners is that you can twist the pipe cleaners together into interesting shapes, such as a snowflake or other design. (Make sure the shape fits inside your jar. )
This is a recording of a recent live teleclass I did with thousands of kids from all over the world. I’ve included it here so you can participate and learn, too! (Click here if you’re looking for the more recent version that also includes Chemical Engineering.)
When you think of slime, do you imagine slugs, snails, and puppy kisses? Or does the science fiction film The Blob come to mind? Any way you picture it, slime is definitely slippery, slithery, and just plain icky — and a perfect forum for learning real science. But which ingredients work in making a truly slimy concoction, and why do they work? Let’s take a closer look…
Materials:
 Did you know you can create a compound microscope and a refractor telescope using the same materials? It’s all in how you use them to bend the light. These two experiments cover the fundamental basics of how two double-convex lenses can be used to make objects appear larger when right up close or farther away.
Did you know you can create a compound microscope and a refractor telescope using the same materials? It’s all in how you use them to bend the light. These two experiments cover the fundamental basics of how two double-convex lenses can be used to make objects appear larger when right up close or farther away.
Things like lenses and mirrors can bend and bounce light to make interesting things, like compound microscopes and reflector telescopes. Telescopes magnify the appearance of some distant objects in the sky, including the moon and the planets. The number of stars that can be seen through telescopes is dramatically greater than can be seen by the unaided eye.
Materials
Helioseismology is the study of wave oscillations in the Sun. By studying the waves, scientists can tell what’s going on inside the Sun. It’s like studying earthquakes to learn what’s going on inside the earth. The Sun is filled with sound, and studying these sound waves is currently the only way scientists can tell what’s going on inside, since the light we see from the Sun is just from the upper surface.
Molecules are vibrating back and forth at fairly high rates of speed, creating waves. Energy moves from place to place by waves. Sound energy moves by longitudinal waves (the waves that are like a slinky). The molecules vibrate back and forth, crashing into the molecules next to them, causing them to vibrate, and so on and so forth. All sounds come from vibrations.
Materials
A meteoroid is a small rock that zooms around outer space. When the meteoroid zips into the Earth’s atmosphere, it’s now called a meteor or “shooting star”. If the rock doesn’t vaporize en route, it’s called a meteorite as soon as it whacks into the ground. The word meteor comes from the Greek word for “high in the air.”
Meteorites are black, heavy (almost twice the normal rock density), and magnetic. However, there is an Earth-made rock that is also black, heavy, and magnetic (magnetite) that is not a meteorite. To tell the difference, scratch a line from both rocks onto an unglazed tile. Magnetite will leave a mark whereas the real meteorite will not.
Materials
Making indoor rain clouds demonstrates the idea of temperature, the measure of how hot or cold something is. Here’s how to do it:
Take two clear glasses that fit snugly together when stacked. (Cylindrical glasses with straight sides work well.)
Fill one glass half-full with ice water and the other half-full with very hot water (definitely an adult job – and take care not to shatter the glass with the hot water!). Be sure to leave enough air space for the clouds to form in the hot glass.
One common misconception is that the seasons are caused by how close the Earth is to the Sun. Today you get to do an experiment that shows how seasons are affected by axis tilt, not by distance from the Sun. And you also find out which planet doesn’t have sunlight for 42 years.
The seasons are caused by the Earth’s axis tilt of 23.4o from the ecliptic plane.
Materials
Many wonders are visible when flying over the Earth at night, especially if you are an astronaut on the International Space Station (ISS)! Passing below are white clouds, orange city lights, lightning flashes in thunderstorms, and dark blue seas. On the horizon is the golden haze of Earth’s thin atmosphere, frequently decorated by dancing auroras as the video progresses. The green parts of auroras typically remain below the space station, but the station flies right through the red and purple auroral peaks. You’ll also see solar panels of the ISS around the frame edges. The wave of approaching brightness at the end of each sequence is just the dawn of the sunlit half of Earth, a dawn that occurs every 90 minutes, as the ISS travels at 5 miles per second to keep from crashing into the earth.
 It just so happens that the Sun’s diameter is about 400 times larger than the Moon, but the Moon is 400 times closer than the Sun. This makes the Sun and Moon appear to be about the same size in the sky as viewed from Earth. This is also why the eclipse thing is such a big deal for our planet. You’re about to make your own eclipses as you learn about syzygy.
It just so happens that the Sun’s diameter is about 400 times larger than the Moon, but the Moon is 400 times closer than the Sun. This makes the Sun and Moon appear to be about the same size in the sky as viewed from Earth. This is also why the eclipse thing is such a big deal for our planet. You’re about to make your own eclipses as you learn about syzygy.
A total eclipse happens about once every year when the Moon blocks the Sun’s light. Lunar eclipses occur when the Sun, Moon, and Earth are lined up in a straight line with the Earth in the. Lunar eclipses last hours, whereas solar eclipses last only minutes.
Materials
You know you’re not supposed to look at the sun, so how can you study it safely? I’m going to show you how to observe the sun safely using a very inexpensive filter. I actually keep one of these in the glove box of my car so I can keep track of certain interesting sunspots during the week!
The visible surface of the sun is called the photosphere, and is made mostly of plasma (remember the grape experiment?) that bubbles up hot and cold regions of gas. When an area cools down, it becomes darker (called sunspots). Solar flares (massive explosions on the surface), sunspots, and loops are all related the sun’s magnetic field. While scientists are still trying to figure this stuff out, here’s the latest of what they do know…
The sun is a large ball of really hot gas – which means there are lots of naked charged particles zipping around. And the sun also rotates, but the poles and the equator move and different speeds (don’t forget – it’s not a solid ball but more like a cloud of gas). When charged particles move, they make magnetic fields (that’s one of the basic laws of physics). And the different rotation rates allow the magnetic fields to ‘wind up’ and cause massive magnetic loops to eject from the surface, growing stronger and stronger until they wind up flipping the north and south poles of the sun (called ‘solar maximum’). The poles flip every eleven years.
There have been several satellites specially created to observe the sun, including Ulysses (launched 1990, studied solar wind and magnetic fields at the poles), Yohkoh (1991-2001, studied x-rays and gamma radiation from solar flares), SOHO (launched 1995, studies interior and surface), and TRACE (launched 1998, studies the corona and magnetic field).
Ok – so back to observing the sun form your own house. Here’s what you need to do:
You might be curious about how to observe the sun safely without losing your eyeballs. There are many different ways to observe the sun without damaging your eyesight. In fact, the quickest and simplest way to do this is to build a super-easy pinhole camera that projects an image of the sun onto an index card for you to view.
CAUTION: DO NOT LOOK AT THE SUN THROUGH ANYTHING WITH LENSES!!
This simple activity requires only these materials:
If you want to get from New York to Los Angeles by car, you’d pull out a map. If you want to find the nearest gas station, you’d pull out a smaller map. What if you wanted to find our nearest neighbor outside our solar system? A star chart is a map of the night sky, divided into smaller parts (grids) so you don’t get too overwhelmed. Astronomers use these star charts to locate stars, planets, moons, comets, asteroids, clusters, groups, binary stars, black holes, pulsars, galaxies, planetary nebulae, supernovae, quasars, and more wild things in the intergalactic zoo.
How to find two constellations in the sky tonight, and how to get those constellations down on paper with some degree of accuracy.
Materials
These are a set of videos made using planetarium software to help you see how the stars and planets move over the course of months and years. See what you think and tell us what you learned by writing your comments in the box below.
You can really feel the Earth rolling around under you as you watch these crazy star trails.
Please login or register to read the rest of this content.
The Moon appears to change in the sky. One moment it’s a big white circle, and next week it’s shaped like a sideways bike helmet. There’s even a day where it disappears altogether. So what gives?
The Sun illuminates half of the Moon all the time. Imagine shining a flashlight on a beach ball. The half that faces the light is lit up. There’s no light on the far side, right? For the Moon, which half is lit up depends on the rotation of the Moon. And which part of the illuminated side we can see depends on where we are when looking at the Moon. Sound complicated? This lab will straighten everything out so it makes sense.
Materials
Using the position of the Sun, you can tell what time it us by making one of these sundials. The Sun will cast a shadow onto a surface marked with the hours, and the time-telling gnomon edge will align with the proper time.
In general, sundials are susceptible to different kinds of errors. If the sundial isn’t pointed north, it’s not going to work. If the sundial’s gnomon isn’t perpendicular, it’s going to give errors when you read the time. Latitude and longitude corrections may also need to be made. Some designs need to be aligned with the latitude they reside at (in effect, they need to be tipped toward the Sun at an angle). To correct for longitude, simply shift the sundial to read exactly noon when indicated on your clock. This is especially important for sundials that lie between longitudinal standardized time zones. If daylight savings time is in effect, then the sundial timeline must be shifted to accommodate for this. Most shifts are one hour.
This is a recording of a recent live teleclass I did with thousands of kids from all over the world. I’ve included it here so you can participate and learn, too
Our solar system includes rocky terrestrial planets (Mercury, Venus, Earth, and Mars), gas giants (Jupiter and Saturn), ice giants (Uranus and Neptune), and assorted chunks of ice and dust that make up various comets and asteroids.
Did you know you can take an intergalactic star tour without leaving your seat? To get you started on your astronomy adventure, I have a front-row seat for you in a planetarium-style star show. I usually give this presentation at sunset during my live workshops, so I inserted slides along with my talk so you could see the pictures better. This video below is long, so I highly recommend doing this with friends and a big bowl of popcorn. Ready?
Please login or register to read the rest of this content.
When I was in grad school, I needed to use an optical bench to see invisible things. I was trying to ‘see’ the exhaust from a new kind of F15 engine, because the aircraft acting the way it shouldn’t – when the pilot turned the controls 20o left, the plane only went 10o. My team had traced the problem to an issue with the shock waves, and it was my job to figure out what the trouble was. (Anytime shock waves appear, there’s an energy loss.)
Since shock waves are invisible to the human eye, I had to find a way to make them visible so we could get a better look at what was going on. It was like trying to see the smoke generated by a candle – you know it’s there, but you just can’t see it. I wound up using a special type of photography called Schlieren.
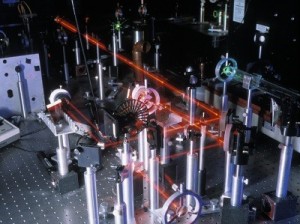 An optical table gives you a solid surface to work on and nails down your parts so they don’t move. This is an image taken with Schlieren photography. This technique picks up the changes in air density (which is a measure of pressure and volume).
An optical table gives you a solid surface to work on and nails down your parts so they don’t move. This is an image taken with Schlieren photography. This technique picks up the changes in air density (which is a measure of pressure and volume).
The air above a candle heats up and expands (increases volume), floating upwards as you see here. The Schlieren technique shines a super-bright xenon arc lamp beam of light through the candle area, bounces it off two parabolic mirrors and passes it through a razor-edge slit and a neutral density filter before reaching the camera lens. With so many parts, I needed space to bolt things down EXACTLY where I wanted them. The razor slit, for example, just couldn’t be anywhere along the beam – it had to be right at the exact point where the beam was focused down to a point.
I’m going to show you how to make a quick and easy optical lab bench to work with your lenses. Scientists use optical benches when they design microscopes, telescopes, and other optical equipment. You’ll need a bright light source like a flashlight or a sunny window, although this bench is so light and portable that you can move it to garage and use a car headlight if you really want to get creative. Once your bench is set up, you can easily switch out filters, lenses, and slits to find the best combination for your optical designs. Technically, our setup is called an optical rail, and the neat thing about it is that it comes with a handy measuring device so you can see where the focal points are for your lenses. Let’s get started:
Please login or register to read the rest of this content.
Hans Lippershey was the first to peek through his invention of the refractor telescope in 1608, followed closely by Galileo (although Galileo used his telescope for astronomy and Lippershey’s was used for military purposes). Their telescopes used both convex and concave lenses.
A few years later, Kepler swung into the field and added his own ideas: he used two convex lenses (just like the ones in a hand-held magnifier), and his design the one we still use today. We’re going to make a simple microscope and telescope using two lenses, the same way Kepler did. Only our lenses today are much better quality than the ones he had back then!
You can tell a convex from a concave lens by running your fingers gently over the surface – do you feel a “bump” in the middle of your hand magnifying lens? You can also gently lay the edge of a business card (which is very straight and softer than a ruler) on the lens to see how it doesn’t lay flat against the lens.
Your magnifier has a convex lens – meaning the glass (or plastic) is thicker in the center than around the edges. The image here shows how a convex lens can turn light to a new direction using refraction. You can read more about refraction here.
A microscope is very similar to the refractor telescope with one simple difference – where you place the focus point. Instead of bombarding you with words, let’s make a microscope right now so you can see for yourself how it all works together. Are you ready?
If you’ve never done this experiment, you have to give it a try! This activity will show you the REAL reason that you should never look at the sun through anything that has lenses in it.
Because this activity involves fire, make sure you do this on a flame-proof surface and not your dining room table! Good choices are your driveway, cement parking lot, the concrete sidewalk, or a large piece of ceramic tile. Don’t do this experiment in your hand, or you’re in for a hot, nasty surprise.
As with all experiments involving fire, flames, and so forth, do this with adult help (you’ll probably find they want to do this with you!) and keep your fire extinguisher handy.
Materials:
Here’s what you need to do:
We’re going to bend light to make objects disappear. You’ll need two glass containers (one that fits inside the other), and the smaller one MUST be Pyrex. It’s okay if your Pyrex glass has markings on the side. Use cooking oil such as canola oil, olive oil, or others to see which makes yours truly disappear. You can also try mineral oil or Karo syrup, although these tend to be more sensitive to temperature and aren’t as evenly matched with the Pyrex as the first choices mentioned above.
Here’s what you need:
Published value for light speed is 299,792,458 m/s = 186,282 miles/second = 670,616,629 mph
Please login or register to read the rest of this content.
Here’s a trick question – can you make the color “yellow” with only red, green, and blue as your color palette? If you’re a scientist, it’s not a problem. But if you’re an artist, you’re in trouble already.
The key is that we would be mixing light, not paint. Mixing the three primary colors of light gives white light. If you took three light bulbs (red, green, and blue) and shined them on the ceiling, you’d see white. And if you could magically un-mix the white colors, you’d get the rainbow (which is exactly what prisms do.)
If you’re thinking yellow should be a primary color – it is a primary color, but only in the artist’s world. Yellow paint is a primary color for painters, but yellow light is actually made from red and green light. (Easy way to remember this: think of Christmas colors – red and green merge to make the yellow star on top of the tree.)
As a painter, you know that when you mix three cups of red, green, and blue paint, you get a muddy brown. But as a scientist, when you mix together three cups of cold light, you get white. If you pass a beam white light through a glass filled with water that’s been dyed red, you’ve now got red light coming out the other side. The glass of red water is your filter. But what happens when you try to mix the different colors together?
The cold light is giving off its own light through a chemical reaction called chemiluminescence, whereas the cups of paint are only reflecting nearby light. It’s like the difference between the sun (which gives off its own light) and the moon (which you see only when sunlight bounces off it to your eyeballs). You can read more about light in our Unit 9: Lesson 1 section.
Here’s what you need:
There are three primary colors of light are red, green, and blue. The three primary colors of paint are red, yellow, and blue (I know it’s actually cyan, yellow, and magenta, which we’ll get to in more detail later, but for now just stick with me and think of the primary colors of paint as red-yellow-blue and I promise it will all make sense in the end).
Most kids understand how yellow paint and blue paint make green paint, but are totally stumped when red light and green light mix to make yellow light. The difference is that we’re mixing light, not paint.
Lots of science textbooks still have this experiment listed under how to mix light: “Stir together one of red water and one glass of green water (dyed with food coloring) to get a glass of yellow water.” Hmmm… the result I get is a yucky greenish-brown color. What happened?
The reason you can’t mix green and red water to get yellow is that you’re essentially still mixing paint, not light. But don’t take our word for it – test it out for yourself with this super-fast light experiment on mixing colors.
Materials:
Ever play with a prism? When sunlight strikes the prism, it gets split into a rainbow of colors. Prisms un-mix the light into its different wavelengths (which you see as different colors). Diffraction gratings are tiny prisms stacked together.
When light passes through a diffraction grating, it splits (diffracts) the light into several beams traveling at different directions. If you’ve ever seen the ‘iridescence’ of a soap bubble, an insect shell, or on a pearl, you’ve seen nature’s diffraction gratings.
Scientist use these things to split incoming light so they can figure out what fuels a distant star is burning. When hydrogen burns, it gives off light, but not in all the colors of the rainbow, only very specific colors in red and blue. It’s like hydrogen’s own personal fingerprint, or light signature.
Astronomers can split incoming light from a star using a spectrometer (you can build your own here) to figure out what the star is burning by matching up the different light signatures.
Materials:
This is the simplest form of camera – no film, no batteries, and no moving parts that can break. The biggest problem with this camera is that the inlet hole is so tiny that it lets in such a small amount of light and makes a faint image. If you make the hole larger, you get a brighter image, but it’s much less focused. The more light rays coming through, the more they spread out the image out more and create a fuzzier picture. You’ll need to play with the size of the hole to get the best image.
While you can go crazy and take actual photos with this camera by sticking on a piece of undeveloped black and white film (use a moderately fast ASA rating), I recommend using tracing paper and a set of eyeballs to view your images. Here’s what you need to do:
Materials:
In this lab, we are going to make an eyeball model using a balloon. This experiment should give you a better idea of how your eyes work. The way your brain actually sees things is still a mystery, but using the balloon we can get a good working model of how light gets to your brain.
Charles Benhamho (1895) created a toy top painted with the pattern (images on next page). When you spin the disk, arcs of color (called “pattern induced flicker colors”) show up around the disk. And different people see different colors!
We can’t really say why this happens, but there are a few interesting theories. Your eyeball has two different ways of seeing light: cones and rods. Cones are used for color vision and for seeing bright light, and there are three types of cones (red, green, and blue). Rods are important for seeing in low light.
One possibility is… Please login or register to read the rest of this content.
Ever notice how BRIGHT your white t-shirt looks in direct sun? That’s because mom washed with fluorescent laundry soap (no kidding!). The soap manufacturers put in dyes that glow white under a UV light, which make your clothes appear whiter than they really are.
Since light is a form of energy, in order for things to glow in the dark, you have to add energy first. So where does the energy come from? There are are few different ways to do this:
When light rays strikes a surface, part of the beam passes through the surface and the rest reflects back, like a ball bouncing on the ground. Where it bounces depends on how you throw the ball.
Have you ever looked into a pool of clear, still water and seen your own face? The surface of the water acts like a mirror and you can see your reflection. (In fact, before mirrors were invented, this was the only way people had to look at themselves.) If you were swimming below the surface, you’d still see your own face – the mirror effect works both ways.
Have you ever broken a pencil by sticking it into a glass of water? The pencil isn’t really broken, but it sure looks like it! What’s going on?
Have you ever wondered why the sky is blue? Or why the sunset is red? Or what color our sunset would be if we had a blue giant instead of a white star? This lab will answer those questions by showing how light is scattered by the atmosphere.
Particles in the atmosphere determine the color of the planet and the colors we see on its surface. The color of the star also affects the color of the sunset and of the planet.
Materials
In this experiment, water is our prism. A prism un-mixes light back into its original colors of red, green, and blue. You can make prisms out of glass, plastic, water, oil, or anything else you can think of that allows light to zip through.
What’s a prism? Think of a beam of light. It zooms fast on a straight path, until it hits something (like a water drop). As the light goes through the water drop, it changes speed (refraction). The speed change depends on the angle that the light hits the water, and what the drop is made of. (If it was a drop of mineral oil, the light would slow down a bit more.) Okay, so when white light passes through a prism (or water drop), changes speed, and turns colors. So why do we see a rainbow, not just one color coming out the other side?
In a simplest sense, a kaleidoscope is a tube lined with mirrors. Whether you leave the end opened or tape on a bag of beads is up to you, but the main idea is to provide enough of an optical illusion to wow your friends. Did you know that by changing the shape and size of the mirrors, you can make the illusion 3D?
If you use only two mirrors, you’ll get a solid background, but add a third mirror and tilt together into a triangle (as shown in the video) and you’ll get the entire field filled with the pattern. You can place transparent objects at the end (like marbles floating in water or mineral oil) or just leave it open and point at the night stars.
The first kaleidoscopes were constructed in 1816 by a scientist while studying polarization. They were quickly picked up as an amusement gadget by the public and have stayed with us ever since.
Materials:
 Imagine you’re a painter. What three colors do you need to make up any color in the universe? (You should be thinking: red, yellow, and blue… and yes, you are right if you’re thinking that the real primary colors are cyan, magenta, and yellow, but some folks still prefer to think of the primary colors as red-yellow-blue… either way, it’s really not important to this experiment which primary set you choose.)
Imagine you’re a painter. What three colors do you need to make up any color in the universe? (You should be thinking: red, yellow, and blue… and yes, you are right if you’re thinking that the real primary colors are cyan, magenta, and yellow, but some folks still prefer to think of the primary colors as red-yellow-blue… either way, it’s really not important to this experiment which primary set you choose.)
Here’s a trick question – can you make the color “yellow” with only red, green, and blue as your color palette? If you’re a scientist, it’s not a problem. But if you’re an artist, you’re in trouble already.
The key is that we would be mixing light, not paint. Mixing the three primary colors of light gives white light. If you took three light bulbs (red, green, and blue) and shined them on the ceiling, you’d see white. And if you could magically un-mix the white colors, you’d get the rainbow (which is exactly what prisms do.)
If you’re thinking yellow should be a primary color – it is a primary color, but only in the artist’s world. Yellow paint is a primary color for painters, but yellow light is actually made from red and green light. (Easy way to remember this: think of Christmas colors – red and green merge to make the yellow star on top of the tree.) It’s because you are using projection of light, not the subtrative combination of colors to get this result.
Here’s a nifty experiment that will really bring these ideas to life (and light!):
Materials:
Why do families share similar features like eye and hair color? Why aren’t they exact clones of each other? These questions and many more will be answered as well look into the fascinating world of genetics!
Genetics asks which features are passed on from generation to generation in living things. It also tries to explain how those features are passed on (or not passed on). Which features are stay and leave depend on the genes of the organism and the environment the organism lives in. Genes are the “inheritance factors “described in Mendel’s laws. The genes are passed on from generation to generation and instruct the cell how to make proteins. A genotype refers to the genetic make-up of a trait, while phenotype refers to the physical manifestation of the trait.
We’re going to create a family using genetics!
Plants need light, water, and soil to grow. If you provide those things, you can make your own greenhouse where you can easily observe plants growing. Here’s a simple experiment on how to use the stuff from your recycling bin to make your own garden greenhouse.
We’ll first look at how to make a standard, ordinary greenhouse. Once your plants start to grow, use the second part of this experiment to track your plant growth. Once you’ve got the hang of how to make a bottle garden, then you can try growing a carnivorous greenhouse.
Please login or register to read the rest of this content.
The way animals and plants behave is so complicated because it not only depends on climate, water availability, competition for resources, nutrients available, and disease presence but also having the patience and ability to study them close-up.
We’re going to build an eco-system where you’ll farm prey stock for the predators so you’ll be able to view their behavior. You’ll also get a chance to watch both of them feed, hatch, molt, and more! You’ll observe closely the two different organisms and learn all about the way they live, eat, and are eaten.