When I was in 10th grade, my teammate and I designed what we thought was pretty clever: a superconductor roller coaster, which we imagined would float effortlessly above its magnetic track. Of course, our roller coaster was only designed on paper, because yttrium barium copper oxide ceramics had only just been discovered by top scientists.
Did you notice how it was smoking in the video? That’s because it was so cold! The usual problem with superconductors is that they need to be incredibly cold in order to exhibit superconductive properties. Yttrium barium copper oxide (YBa2Cu3O7) was the first compound that used liquid nitrogen for cooling, making superconductors a lot less expensive to work with – you no longer needed a cryogenic lab in order to levitate objects above a magnet.
Recently, scientists have found a way to make an amazing superconductor by taking a single crystal sapphire wafer and coating it with a thin (~1µm thick) ceramic material (yttrium barium copper oxide). Normally, the ceramic layer has no interesting magnetic or electrical properties, but that’s when you’re looking at it at room temperature. If you cool this material below -185ºC (-301ºF), it turns out that the ceramic material becomes a superconductor, meaning that it conducts electricity without resistance, with no energy loss. Zero. That’s what makes it a ‘superconductor’.
 To further understand superconductivity, it’s helpful to understand what normally happens to electricity as it flows through a wire. As you may know, energy cannot be created or destroyed, but can be changed from one form to another.
To further understand superconductivity, it’s helpful to understand what normally happens to electricity as it flows through a wire. As you may know, energy cannot be created or destroyed, but can be changed from one form to another.
In the case of wires, some of the electrical energy is changed to heat energy. If you’ve ever touched a wire that had been in use for a while, and discovered it was hot, you’ve experienced this. The heat energy is a waste. It simply means that less electricity gets to its final destination.
This is why superconductivity is so cool (no pun intended.) By cooling things down to temperatures near absolute zero, which is as low as temperatures can get, you can create a phenomenon where electricity flows without having any of it converted to heat.
Why do superconductors float above magnets?
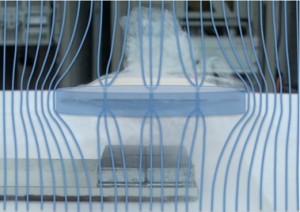 Scientists also figured out that superconductors and magnetic field really do not like each other. The Meissner effect happens when a superconductor expels all its magnetic fields from inside.
Scientists also figured out that superconductors and magnetic field really do not like each other. The Meissner effect happens when a superconductor expels all its magnetic fields from inside.
However, if you make your superconductor thin enough, you can get the magnetic field to penetrate in discrete quantities (this is real quantum physics now) called flux tubes (the blue lines that go through the disc).
Inside each of the magnetic flux tubes, the superconductivity is destroyed, but the superconductor tries to keep the magnetic tubes pinned in weak areas and any movement of the superconductor itself (like if you pushed it) causes the flux tubes to move, and this is what traps (or locks) the superconductor in midair.
If you’d like to experiment with superconductors yourself, check out this information.


 Make yourself a grab bag of fun things to test: copper pieces (nails or pipe pieces), zinc washers, pipe cleaners, Mylar, aluminum foil, pennies, nickels, keys, film canisters, paper clips, load stones (magnetic rock), other rocks, and just about anything else in the back of your desk drawer.
Make yourself a grab bag of fun things to test: copper pieces (nails or pipe pieces), zinc washers, pipe cleaners, Mylar, aluminum foil, pennies, nickels, keys, film canisters, paper clips, load stones (magnetic rock), other rocks, and just about anything else in the back of your desk drawer. When you turn on a switch, it’s difficult to really see what’s going on… which is why we make our own from paperclips, brass fasteners, and index cards.
When you turn on a switch, it’s difficult to really see what’s going on… which is why we make our own from paperclips, brass fasteners, and index cards.
 This simple FET circuit is really an electronic version of the
This simple FET circuit is really an electronic version of the 
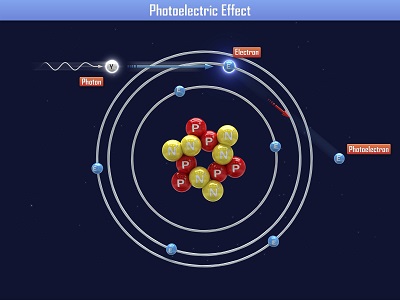 Einstein received a Nobel Prize for figuring out what happens when you shine blue light on a sheet of metal. When he aimed a blue light on a metal plate, electrons shot off the surface. (Metals have electrons which are free to move around, which is why metals are electrically conductive. More on this in Unit 10).
Einstein received a Nobel Prize for figuring out what happens when you shine blue light on a sheet of metal. When he aimed a blue light on a metal plate, electrons shot off the surface. (Metals have electrons which are free to move around, which is why metals are electrically conductive. More on this in Unit 10).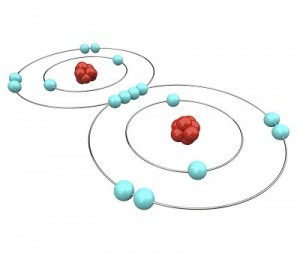
 Let’s go a little further with this shell thing. An atom can have as few as one and as many as seven shells. Imagine our balloon again. Now there can be a balloon inside of a balloon inside a balloon and so on. Up to seven balloons! Each balloon, whoops, I mean shell, can have only so many electrons in it. This simple equation 2n2 tells you how many electrons can be in each shell. The n stands for the number of the shell.
Let’s go a little further with this shell thing. An atom can have as few as one and as many as seven shells. Imagine our balloon again. Now there can be a balloon inside of a balloon inside a balloon and so on. Up to seven balloons! Each balloon, whoops, I mean shell, can have only so many electrons in it. This simple equation 2n2 tells you how many electrons can be in each shell. The n stands for the number of the shell.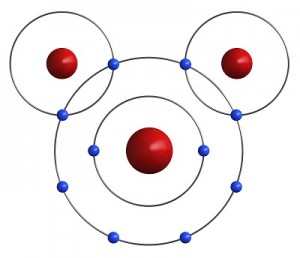 Luckily, two Hydrogen atoms happen by. Each one of them has only one electron in its outer shell and needs one more to be satisfied. If both Hydrogens share their one electron with the Oxygen, the oxygen has eight electrons and is satisfied. Also, if the Oxygen shares an electron with each Hydrogen, then both Hydrogens are satisfied as well. Just like your mother told you, it’s nice to share. It is this sharing of electrons that makes atoms come together to form molecules.
Luckily, two Hydrogen atoms happen by. Each one of them has only one electron in its outer shell and needs one more to be satisfied. If both Hydrogens share their one electron with the Oxygen, the oxygen has eight electrons and is satisfied. Also, if the Oxygen shares an electron with each Hydrogen, then both Hydrogens are satisfied as well. Just like your mother told you, it’s nice to share. It is this sharing of electrons that makes atoms come together to form molecules.

