Let’s try another way to look at this. You’re playing miniature golf and you come to the old wind mill hole. Your friend takes a shot and since the blades of the windmill are going nice and slow he gets the ball right through. Now it’s your turn. Suddenly you hear a zap and a pow and sparks go flying. Something has gone wrong with the wind mill and it starts spinning at amazing speeds. You decide to give it a try and hit the ball towards the wind mill.
Well since it is spinning out of control, those blades now form almost a solid disk so that there is no way your ball can get through the wind mill. Electrons do the same thing. They move so fast that even though there may not be many of them, they form a shell that can’t be penetrated. (To be clear, particles that are smaller than an atom can go through the shells and pop out the other side.)
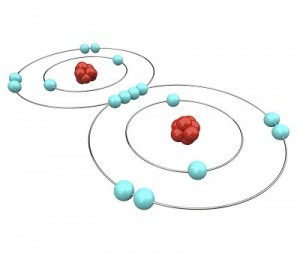
 Let’s go a little further with this shell thing. An atom can have as few as one and as many as seven shells. Imagine our balloon again. Now there can be a balloon inside of a balloon inside a balloon and so on. Up to seven balloons! Each balloon, whoops, I mean shell, can have only so many electrons in it. This simple equation 2n2 tells you how many electrons can be in each shell. The n stands for the number of the shell.
Let’s go a little further with this shell thing. An atom can have as few as one and as many as seven shells. Imagine our balloon again. Now there can be a balloon inside of a balloon inside a balloon and so on. Up to seven balloons! Each balloon, whoops, I mean shell, can have only so many electrons in it. This simple equation 2n2 tells you how many electrons can be in each shell. The n stands for the number of the shell.
The first shell can have up to 2 x 1(first shell)2 or 2 electrons. The second shell can have up to 2 x 2(second shell)2 or 8 electrons. The third shell can have up to 2 x 32 or 18 electrons. The fourth shell can have up to 2 x 42 or 32 electrons. All the way up to the seventh shell which can have 2 x 72 or 98 electrons!
One last thing about shells, the shells have to be full before the electrons will go to the next shell. A helium atom will have two electrons. Both of them will be in the first shell. A Lithium atom will have three electrons. Two will be in the first shell and one (since the first shell is filled) will be in the second shell.
Electrons provide the size and stability of the atom and, as such, the mass and the structure of all matter. Electrons are also the key to all electromagnetic energy. But wait, that’s not all! It is the number of electrons in an atom that determines if and how atoms come together to form molecules. Electrons determine how and what matter will be.
Atoms like to feel satisfied and they feel satisfied if they are “full”. An atom is “full” if its outer electron shell has as many electrons as it can hold or if there are eight or a multiple of eight (16, 24 etc.) electrons in the outer shell. This is called the octet rule and works most of the time, but is not perfect.
If an atom is not full, it is not satisfied. An unsatisfied atom needs to do something with its electrons to be happy. Luckily atoms are very friendly and love to share. Most atoms are not satisfied as individuals. The oxygen atom has six electrons in its outer shell. It needs eight electrons to be satisfied.
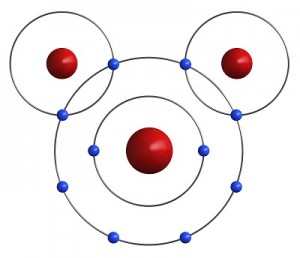 Luckily, two Hydrogen atoms happen by. Each one of them has only one electron in its outer shell and needs one more to be satisfied. If both Hydrogens share their one electron with the Oxygen, the oxygen has eight electrons and is satisfied. Also, if the Oxygen shares an electron with each Hydrogen, then both Hydrogens are satisfied as well. Just like your mother told you, it’s nice to share. It is this sharing of electrons that makes atoms come together to form molecules.
Luckily, two Hydrogen atoms happen by. Each one of them has only one electron in its outer shell and needs one more to be satisfied. If both Hydrogens share their one electron with the Oxygen, the oxygen has eight electrons and is satisfied. Also, if the Oxygen shares an electron with each Hydrogen, then both Hydrogens are satisfied as well. Just like your mother told you, it’s nice to share. It is this sharing of electrons that makes atoms come together to form molecules.
Click here to go to next lesson on Electrostatic Charge.
[/am4show]

Oh gosh – I think you caught an algebraic answer! Let me redo this section of the video so it’s correct. Thanks for your eagle eye!
Quick comment on math in the video .. At approx 12:10 into the video, the “square” seems to be dropped from the radius in the equation, yielding a larger than expected final value for the height of the “soap cylinder.” Calculating using r^2 in the denominator, we got an answer of 4.73*10^-5 in. Is that right?