We’re going to learn about saturated solutions today! A solution with solute (the powder added in) that dissolves until it leaves bits at the bottom (indicating that it cannot dissolve anymore). An unsaturated solution is the solution before the bits start showing up at the bottom, meaning that there’s enough solvent to hold all the solute you add in.
Please login or register to read the rest of this content.
Sodium chloride, ammonium chloride, sodium carbonate, and many others have tiny charged particles (positive and negative) called “salts”. When a “salt” is dissolved in water, it will separate into the two particles (plus and minus), which means that if you pass a current through the solution, the positive particles (positive ions) become attracted to the negative pole and the negative particles (negative ions) move toward the positive pole. This movement is allowing electricity to flow, and the this is the reason that “salt” solutions conduct electricity.
Please login or register to read the rest of this content.
We’re using the solution from the last experiment (the iron in what used to be a copper sulfate solution) for part of this experiment. This experiment is tricky to see a color change, which is why we’re going to look at the paper towel for the telltale blue that will indicate the presence of iron.
Please login or register to read the rest of this content.
We’re going to look at how iron reacts with an acid and detect the iron bits with an indicator. We’re going to do several experiments that will need a bit more time, like overnight, so be sure to store this experiment out of reach of small kids while you are waiting for it to progress.
Please login or register to read the rest of this content.
We’re going to learn about the properties of combustion by doing a simple set of experiments. Because this involves FIRE, please make sure you have an adult handy with you while you do your experiments. We’re going to learn how to detect the presence of carbon dioxide by looking for a “precipitate” – tiny little particles that seem to form out of nowhere.
Please login or register to read the rest of this content.
This is a neat set of experiments, and the trick works because carbon dioxide is heavier than air so it really can be “poured” just like a liquid. The problem is that most people mis-judge the “pour”, so if you want to practice first, capture the smoke from an extinguished candle first (get an adult to help you!) and practice pouring a gas into a bowl.
Please login or register to read the rest of this content.
This experiment is in two parts. We’re going to use chemistry to separate mixtures. We’re going to use a mixture we prepared in the previous experiment. Also, please make sure you displose of the copper sulfate correctly, you can’t simply just throw it in the trash or down the drain.
Please login or register to read the rest of this content.
Water is measured in inches or centimetres when it’s in a test tube. We’re going to make a solution that we will be keeping for not only today’s but also future experiments as well. The solution is hazardous to aquatic life, so make sure you watch all the disposal instructions near the end of the video.
You’re going to create a beautiful blue color by combining two substances together, one is an indicator for the other (the iron).
Please login or register to read the rest of this content.
You’ll find carbon dioxide everywhere: when you exhale, the bubbles in your soda, in Venus’ atmosphere… The next set of experiments bounce around a little within the manual that came with your set of chemicals. We put all of them together here because it makes the most sense – watch and you’ll see!
It’s hard to know what molecules are present in a solution, so with litmus and limewater, you now have ways to detect these! This is only the start… let’s watch the video now to learn more.
Please login or register to read the rest of this content.
Litmus is from a plant, so it will have a limited shelf life (you’ll notice a different, more earthy smell to it). The amount of dry powder provided in the kit is enough for three solutions, more than enough for our experiments. If you notice particles and sludge at the bottom of your container, it’s totally normal, and all you need to do is pour the liquid off and scrape out the residue and throw (the residue) away. If you add a little denatured alcohol (ethyl alcohol), the solution will last a bit longer on the shelf.
We’re going to do a number of experiments with this solution, all in one video. My friend, a PhD Chemistry professor, helped make a new set of videos for you that will walk you through every step.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Sparks flying off in all directions…that’s fun. In this lab, we will show how easy it is to produce those shooting sparks. In a sparkler you buy at the store, the filings used are either iron or aluminum.
The filings are placed in a mixture that, when dry, adheres to the metal rod or stick that is used in making the sparkler. The different colors are created by adding different powdered chemicals to the mixture before it dries. When they burn, we get red, blue, white, and green.
Please login or register to read the rest of this content.
Click here to go to next lesson on Limiting Reactants.
A lot of chemical reactions happen in a solution (it allows the chemicals to interact much more easily with each other when it is), so chemists define how much of the solute is in the solution by the term MOLARITY.
Molarity is a really convenient unit of concentration and it works like this. If I have 10 moles of solute in 10 liters of water, what’s the molarity? 10/10 = 1! So it’s a 1M solution. What if I have 20 moles in 10 liters? Then it’s a 2M solution. See how easy that is?
Please login or register to read the rest of this content.
Click here to go to next lesson on Iron Sparklers.
Precipitate reactions are like watching a snow globe, but the snow appears out of nowhere.
For example, you can combine two liquid solutions that are totally clear and when you put them together, they each break apart into ions and then recombine in a way that looks like white snow in your test tube. Basically precipitate reactions make it possible to see the ions in a solution because they form a salt that’s not soluble – it doesn‘t dissolve in the solution. You can also get different colors of the precipitate snow, depending on which reactants you start out with. If you were to use potassium bromide (KBr) with silver nitrate, you’d find a yellowish snowstorm of silver bromide (AgBr).
Please login or register to read the rest of this content.
Click here to go to next lesson on Electrolytes.
We’re going to be mixing up dinosaur toothpaste, doing experiments with catalysts, discovering the 5 states of matter, and building your own chemistry lab station as we cover chemical kinetics, phase shifts, the states of matter, atoms, molecules, elements, chemical reactions, and much more. We’re also going to turn liquid polymers into glowing putty so you can amaze your friends when it totally glows in the dark. AND make liquids freeze by heating them up (no kidding) using a scientific principle called supercooling,
Materials:
- Chemistry Worksheet
- Aluminum pie plate
- Bowl
- Clear glue or white glue
- Disposable cups
- Goggles & gloves
- Hydrogen peroxide
- OPTIONAL: Instant reusable hand warmer (containing sodium acetate )
- Liquid soap
- Popsicle sticks
- Scissors or pliers
- Sodium tetraborate (also called “Borax”)
- Water bottle
- Yeast
- Yellow highlighter
- Optional: If you want to see your experiments glow in the dark, you'll need a fluorescent UV black light (about $10 from the pet store - look in cleaning supplies under "Urine-Off" for a fluorescent UV light). UV flashlights and UV LEDs will not work.
This experiment is for advanced students. Hydrolysis is a chemical reaction that involves breaking a molecular bond using water. In chemistry, there are three different types of hydrolysis: sat hydrolysis, acid hydrolysis, and base hydrolysis. In nature, living organisms survive by making their energy from processing food. The energy converted from food is stored in ATP molecules. To release the energy stored in food, a phosphate group breaks off an ATP molecule (and becomes ADP) using hydrolysis and releases energy from the bonds.
Please login or register to read the rest of this content.
This is a recording of a recent live teleclass I did with thousands of kids from all over the world. I’ve included it here so you can participate and learn, too! (Click here if you’re looking for the more recent version that also includes Chemical Engineering.)
When you think of slime, do you imagine slugs, snails, and puppy kisses? Or does the science fiction film The Blob come to mind? Any way you picture it, slime is definitely slippery, slithery, and just plain icky — and a perfect forum for learning real science. But which ingredients work in making a truly slimy concoction, and why do they work? Let’s take a closer look…
Materials:
- Sodium tetraborate (also called “Borax” – it’s a laundry whitener) – about 2 tablespoons
- Clear glue or white glue (clear works better if you can find it) – about 1/2 cup
- Yellow highlighter
- Pliers or sharp razor (with adult help). (PREPARE: Use this to get the end off your highlighter before class starts so you can extract the ink-soaked felt inside. Leave the felt inside highlighter with the end loosely on (so it doesn’t dry out))
- Resuable Instant Hand Warmer that contains sodium acetate (Brand Name: EZ Hand Warmer) – you’ll need two of these
- Scissors
- Glass half full of COLD water (PREPARE: put this in the fridge overnight)
- Mixing bowl full of ice (PREPARE: leave in freezer)
- Salt
- Disposable aluminum pie place or foil-wrapped paper plate
- Disposable cups for solutions (4-6)
- Popsicle sticks for mixing (4-6)
- Rubber gloves for your hands
- Optional: If you want to see your experiments glow in the dark, you’ll need a fluorescent UV black light (about $10 from the pet store – look in cleaning supplies under “Urine-Off” for a fluorescent UV light). UV flashlights and UV LEDs will not work.
Chemistry is all about studying chemical reactions and the combinations of elements and molecules that combine to give new stuff. Chemical reactions can be written down as a balanced equation that shows how much of each molecule and compound are needed for that particular reaction. Here’s how you do it:
Please login or register to read the rest of this content.
If you’re into magic shows, this is a good one to perform for an audience, because the solution goes from purple to pink to green to blue and back again!
Le Chatelier’s principle states that when the temperature is raised, an equilibrium will shift away from the side that contains energy. When temperature is lowered, the reaction shifts toward the side that contains the energy. That’s a little hard to understand, so that’s why there’s a really cool experiment that will show you exactly what we see happening with this principle.
Remember that exothermic reactions are chemical reactions that give off energy. In this experiment, this reaction is exothermic, which is going to be an important key in predicting which way the system will balance itself as it gets subjected to temperature changes.
Please login or register to read the rest of this content.
We’re going to do an experiment where it will look like we can boil soda on command… but the truth is, it’s not really boiling in the first place! If you drink soda, save one for doing this experiment. Otherwise, get one that’s “diet” (without the sugar, it’s a lot easier to clean up).
Please login or register to read the rest of this content.
Molecules are the building blocks of matter.
You’ve probably heard that before, right? But that does it mean? What does a molecule look like? How big are they?
While you technically can measure the size of a molecule, despite the fact it’s usually too small to do even with a regular microscope, what you can’t do is see an image of the molecule itself. The reason has to do with the limits of nature and wavelengths of light, not because our technology isn’t there yet, or we’re not smart enough to figure it out. Scientists have to get creative about the ways they do about measuring something that isn’t possible to see with the eyes.
Here’s a cool experiment you can do that will approximate the size of a molecule. Here’s what you need:
Please login or register to read the rest of this content.
Chemical Data & Safe Handling Information Sheet
What do I really need to know first? First of all, the chemicals in this set should be stored out of reach of pets and children. Grab the chemicals right now and stuff them in a safe place where accidents can’t happen. Do this NOW!
When you’re done storing your chemicals out of reach, watch this video:
Please login or register to read the rest of this content.
Chemical Data & Safe Handling Information Sheet
What do I really need to know first? First of all, the chemicals in this set should be stored out of reach of pets and children. Grab the chemicals right now and stuff them in a safe place where accidents can’t happen. Do this NOW!
When you’re done storing your chemicals out of reach, watch this video:
Please login or register to read the rest of this content.
You can go your whole life without paying any attention to the chemistry behind acids and bases. But you use acids and bases all the time! They are all around you. We identify acids and bases by measuring their pH.
Every liquid has a pH. If you pay particular attention to this lab, you will even be able to identify most acids and bases and understand why they do what they do. Acids range from very strong to very weak. The strongest acids will dissolve steel. The weakest acids are in your drink box. The strongest bases behave similarly. They can burn your skin or you can wash your hands with them.
Acid rain is one aspect of low pH that you can see every day if you look for it. This is a strange name, isn’t it? We get rained on all the time. If people were dissolving, if the rain made their skin smoke and burn, you’d think it would make headlines, wouldn’t you? The truth is acid rain is too weak to harm us except in very rare and localized conditions. But it’s hard on limestone buildings.
Please login or register to read the rest of this content.
Magnesium is one of the most common elements in the Earth’s crust. This alkaline earth metal is silvery white, and soft. As you perform this lab, think about why magnesium is used in emergency flares and fireworks. Farmers use it in fertilizers, pharmacists use it in laxatives and antacids, and engineers mix it with aluminum to create the BMW N52 6-cylinder magnesium engine block. Photographers used to use magnesium powder in the camera’s flash before xenon bulbs were available.
Most folks, however, equate magnesium with a burning white flame. Magnesium fires burn too hot to be extinguished using water, so most firefighters use sand or graphite.
We’re going to learn how to (safely) ignite a piece of magnesium in the first experiment, and next how to get energy from it by using it in a battery in the second experiment. Are you ready?
Please login or register to read the rest of this content.
In this lab, we’re going to investigate the wonders of electrochemistry. Electrochemistry became a new branch of chemistry in 1832, founded by Michael Faraday. Michael Faraday is considered the “father of electrochemistry”. The knowledge gained from his work has filtered down to this lab. YOU will be like Michael Faraday. I imagined he would have been overjoyed to do this lab and see the results. You are soooo lucky to be able to take an active part in this experiment. Here’s what you’re going to do…
You will be “creating” metallic copper from a solution of copper sulfate and water, and depositing it on a negative electrode. Copper is one of our more interesting elements. Copper is a metal, and element 29 on your periodic table. It conducts heat and electricity very well.
Many things around you are made of copper. Copper wire is used in electrical wiring. It has been used for centuries in the form of pipes to distribute water and other fluids in homes and in industry. The Statue of Liberty is a wonderful example of how beautiful 180,000 pounds of copper can be. Yes, it is made of copper, and no, it doesn’t look like a penny…..on the surface. The green color is copper oxide, which forms on the surface of copper exposed to air and water. The oxide is formed on the surface and does not attack the bulk of the copper. You could say that copper oxide protects the copper.
Please login or register to read the rest of this content.
If we don’t have salt, we die. It’s that simple. The chemical formula for salt is NaCl. Broken down, we have Na (sodium) and Cl (chlorine). Either one of these can be fatal in sufficient quantities. When chemically combined, these two deadly elements become table salt. What once could kill now keeps us alive. Isn’t chemistry awesome?
Chlorine, element 17, is called a halogen as are all the elements in the 17th row. All halogens have similar chemical properties. They are highly reactive nonmetals, and react easily with most metals. Sodium is a metal, and is bonded with sodium in the table salt used in this lab. Besides being found in salt, chlorine has many uses in our world such as killing bacteria in our water, making plastic, cleaning products, and the list goes on. A very useful chemical, and is among the top ten chemicals produced in the United States. Ever since its discovery in 1774, chlorine has been very useful. It is found in nature in sodium chloride, but in very small concentrations. Seawater, the most abundant source of chlorine, has a concentration of only 19g of chlorine per liter.
Please login or register to read the rest of this content.
Electricity. Chemistry. Nothing in common, have nothing to do with each other. Wrong! Electrochemistry has been a fact since 1774. Once electricity was applied to particular solutions, changes occurred that scientists of the time did not expect.
In this lab, we will discover some of the same things that Farraday found over 300 years ago. We will be there as things tear apart, particles rush about, and the power of attraction is very strong. We’re not talking about dancing, we’re talking about something much more important and interesting….we’re talking about ELECTROCHEMISTRY!
Please login or register to read the rest of this content.
Ammonia has been used by doctors, farmers, chemists, alchemists, weightlifters, and our families since Roman times. Doctors revive unconscious patients, farmers use it in fertilizer, alchemists tried to use it to make gold, weightlifters sniff it into their lungs to invigorate their respiratory system and clear their heads prior to lifting tremendous loads. At home, ammonia is used to clean up the ketchup you spilled on the floor and never cleaned up.
The ammonia molecule (NH3) is a colorless gas with a strong odor – it’s the smell of freshly cleaned floors and windows. Mom is not cleaning with straight ammonia (it’s gas at room temperature because it boils at -28oF, so the stuff she cleans with is actually ammonium hydroxide, a solution of ammonia and water). Ammonia is found when plants and animals decompose, and it’s also in rainwater, volcanoes, your kidneys (to neutralize excess acid), in the ocean, some fertilizers, in Jupiter’s lower cloud decks, and trace amounts are found in our own atmosphere (it’s lighter than air).
Please login or register to read the rest of this content.
This experiment is for advanced students.
Purple and white colors, making the whitewash that Tom Sawyer used, and produce an exothermic chemical reaction…..does it get any better?
Limewater is one of the compounds we work with in this experiment. Limewater was used in the old days of America. We’re talking about the 80’s…..the 1880’s.
Traveling medicine shows sold what was called “patent medicines”. These usually had no medicinal properties at all. The man in charge, the salesman of the operation, was called a “huckster”. He would have the one of the people gathered around to listen to him blow into limewater. Their exhaled breath contains carbon dioxide, and the lime water turned cloudy, just like in our experiment.
The man would hold up the glass with the cloudy limewater in it and pour in some of his fantastic remedy. As long as the “medicine” was acidic, it would turn the cloudy limewater clear. This was proof that the remedy would cure whatever ailed the person.
Please login or register to read the rest of this content.
This experiment is for advanced students. This is a repeat of the experiment: Can Fish Drown? but now we’re going to do this experiment again with your new chemistry glassware.
The aquarium looked normal in every way, except for the fish. They were breathing very fast and sinking head first to the bottom of the tank. They would sink a few inches, then jerk into proper movement again.
The student had to figure out what was wrong. He had set up the aquarium as an ongoing science project, and it was his responsibility to maintain the fish tank. His grade depended on it.
He went to his mom for help. She looked over the setup. “Have you tested the water?”
A quizzical look on his face, the boy said, “Everything is normal nitrates, nitrites, hardness, alkalinity, and pH. The pH was a little acidic, but not outside the proper range.”
Please login or register to read the rest of this content.
This experiment is for advanced students.
Don’t put this in your car….yet. Hydrogen generation, capture, and combustion are big deals right now. The next phase of transportation, and a move away from fossil fuels in not found in electric cars. Electric cars are waiting until hydrogen fuel cell vehicles become practical. It can be done and is being done.
Cars being powered by hydrogen are here, but not on the market yet. Engineers and chemists are always finding new ways to improve the chemical reaction that produces hydrogen and making the vehicles more efficiently use the fuel. Hydrogen fuel is not just easy to make, it is inexpensive, and the “exhaust” is water.
We will generate hydrogen in this lab. We will also see how combustible it is. Just let your imagination wander….just a bit and you will see noiseless cars and trucks zipping along the streets and interstates, carrying people and cargo. The Indianapolis 500 wouldn’t be quite the same, though. “And there they go, roaring, I mean quietly entering turn two…”
Please login or register to read the rest of this content.
This experiment is for advanced students.
In industry, hydrogen peroxide is used in paper making to bleach the pulp before they form it into paper. Biologists, when preparing bones for display, use peroxide to whiten the bones.
At home, 3% peroxide combined with ammonium hydroxide is used to give dark-haired people their desired blonde moment. Peroxide is also used on wounds to clean them and remove dead tissue. Peroxide slows the flow from small blood vessels and oozing in wounds as well.
Please login or register to read the rest of this content.
This experiment is for advanced students.
This time we’re going to use a lot of equipment… really break out all the chemistry stuff. We’ll need all this stuff to generate oxygen with potassium permanganate (KMNO4). We will work with this toxic chemical and we will be careful…won’t we?
Please login or register to read the rest of this content.
This experiment is for advanced students.
Zinc (Zn), is a metal and it is found as element #30 on the periodic table. We need a little zinc to keep our bodies balanced, but too much is very dangerous.
Zinc is just like the common, everyday substance that we all know as di-hydrogen monoxide (which is the chemical name for water). We need water to survive, but too much will kill us.
DHMO: In chemistry, “Di” equals the number 2; hydrogen is H; mono equals the number one; and oxide is derived from oxygen, and its symbol is O. Put these together and you have Di-hydrogen (H2), and mono oxygen (O). Put them together, what do you have? Water!
Please login or register to read the rest of this content.
This experiment is for advanced students.
Lewis and Clark did this same experiment when they reached the Oregon coast in 1805. Men from the expedition traveled fifteen miles south of the fort they had built at the mouth of the Columbia River to where Seaside, Oregon now thrives.
In 1805, however, it was just men from the fort and Indians. They built an oven of rocks. For six weeks, they processed 1,400 gallons of seawater, boiling the water off to gain 28 gallons of salt.
Please login or register to read the rest of this content.
This experiment is for advanced students. This lab builds on concepts from the previous carbon dioxide lab.
Limewater….carbon dioxide…indicators. We don’t know too much about these things. Sure, we know a little. Carbon dioxide is exhaled by us and plants need it to grow. Burning fossil fuels produces carbon dioxide.
Indicators…something we observe that confirms to us that something specific is happening. Lime water turns cloudy and forms a precipitate in the presence of carbon dioxide. Blue litmus paper turns red in the presence of an acid. The dog barking at the door and dancing around indicates that you better let the dog out, and quick, to avoid….a pet spill?
Please login or register to read the rest of this content.
This experiment is for advanced students.
Who gets to burn something today? YOU get to burn something today!
You will be working with Zinc (Zn). Other labs in this kit allow us to burn metal, but there is a bit of a twist this time. We will be burning a powder.
Why a powder instead of a solid ribbon or foil as in the other labs? Have you heard of surface area being a factor in a chemical reaction? The more surface area there is to burn, the more dramatic the chemical change. So, with this fact in mind, a powder should burn faster or be more likely to burn than a large solid.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Brimstone is another name for sulfur, and if you’ve ever smelled it burn…..whoa….I’m telling you ….you will see for yourself in this lab. It is quite a smell, for sure. Sulfur is element #6 on the periodic table. Sulfur is used in fertilizer, black powder, matches, and insecticides. In pioneer times sulfur was put into patent medicines and used as a laxative.
To further the evil reputation of sulfur, or brimstone, when sulfur is burned in a coal fired power plant, sulfur dioxide is produced. The sulfur is spewed into the air, where it is reacts with moisture in the air to form sulfuric acid. The clouds get full and need to let go of this sulfuric acid. Down comes the acid rain to wreak havoc on the masonry and plant life below.
Please login or register to read the rest of this content.
This experiment is for advanced students.
ACID!!! The word causes fear to creep in and get our attention.
BASIC!!! The word causes nothing to stir in most of us.
The truth is, a strong acid (pH 0-1) is dangerous, but a strong basic (pH 13-14) is just as dangerous. In this lab, we will get comfortable with the basics of bases and the acidity of acids along with how you can use both and tell the difference between them.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Ever use soap? Sodium hydroxide (NaOH) is the main component in lye soap. NaOH is mixed with some type of fat (vegetable, pig, cow, etc). Scent can be added for the ‘pretty’ factor and pumice or sand can be added for the manly “You’re coming off my hands and I’ll take no guff” factor. Lots of people still make their own soap and they enjoy doing it.
Please login or register to read the rest of this content.
Potassium permanganate (KMnO4) in water turns an intense, deep, purple. It is important in the film industry for aging props and clothing to make them look much older than they are.
Also, artists use it in bone carving. People who carve antlers and bone use KMnO4 to darken the surface of the bone to make it look aged. They make the carving, soak it in potassium permanganate, then carve more, and repeat. The end result is a carving that has a light golden brown color. More dipping will darken the carving even more.
Potassium permanganate is going to undergo a chemical change with this activity. In this experiment, we will be able to witness several indicators of chemical change. Color changes, bubbles from gas generation, temperature change, and color disappearance are all indicators of chemical changes.
Please login or register to read the rest of this content.
How do you know if your brother is stealing your candy? Unwrap a wrapped hard candy that he likes a lot. Roll the candy around in the powdered food dye that matches the candy. (Push the powder into the candy so it “disappears”.) Re-wrap the candy. Set the candy in the place where it usually disappears from. Wait ten minutes after the candy disappears. Find your brother. He will be sporting a new color on his hands and mouth. Dye is hard to remove. It will have to be worn every day at school until it fades away as the skin cells slough off. The dye he now wears is in indicator that he has been taking your candy.
Please login or register to read the rest of this content.
In gas form, element #59 is deadly. However, when iodine is in liquid form, it helps heal cuts and scrapes. The iodine molecule occurs in pairs, not as a single atom (many halogens do this, and it's called a diatomic molecule). It's hard to find iodine in nature, though it's essential for staying healthy... too little iodine in the body takes a heavy toll on how well the brain operates.
A chunk of iodine is blackish-blue, and will sublimate (go from a solid straight to a gas). Iodine is the heaviest element needed by living things. Iodized salt is sodium chloride fortified with iodine to prevent people from not getting enough iodine in their daily diets.
Iodine is found in seaweed (kelp) and seafood as well as vegetables that are grown in dirt that has high iodine levels. People that live inland and do not eat fish often have lower iodine levels than their coastal, fish-eating neighbors. The trick is not to get too much or too little iodine in your diet, because the symptoms of deficiency and excess levels are quite similar.
Starch (like cornstarch) are used as an indicator for detecting iodine in chemistry experiments. When combined with iodine, starch forms a blue-black color in the solution. We're going to do this and many other activities in this lab, because this experiment is actually several labs rolled into one. First, we have to make iodine, store it, and then we get to use it in several experiments. Are you ready?
Please login or register to read the rest of this content.
This experiment is for advanced students.
Zinc and Hydrogen are important elements for all of us. Zinc (Zn) metal is element #30 on the periodic table. Lack of zinc in our diets will delay growth of our bodies and can kill.
Hydrogen gas (H) is element #1 on the periodic table. Hydrogen was discovered in the 1500s. In a pure state, hydrogen combustion (in small quantities) is interesting. In large amounts, mixed with oxygen, the explosion can be devastating.
Please login or register to read the rest of this content.
We've created a video that shows you how to safely do this experiment, although if you're nervous about doing this one, just watch the video and skip the actual experiment.
Bromine is a particularly nasty chemical, so be sure to very carefully follow the steps we've outlined in the video. You MUST do this experiment outdoors. We'll be making a tiny amount to show how the chemical reactions involving bromine work.
Please login or register to read the rest of this content.
WARNING!! THIS EXPERIMENT IS PARTICULARLY DANGEROUS!! (No kidding.) This experiment is for advanced students.
We’ve created a video that shows you how to safely do this experiment, although if you’re nervous about doing this one, just watch the video and skip the actual experiment.
The gas you generate with this experiment is lethal in large doses, so you MUST do this experiment outdoors. We’ll be making a tiny amount to show how the chemical reactions of chlorine and hydrogen work.
Please login or register to read the rest of this content.
Chemical Data & Safe Handling Information Sheet
What do I really need to know first? First of all, the chemicals in this set should be stored out of reach of pets and children. Grab the chemicals right now and stuff them in a safe place where accidents can’t happen. Do this NOW!
If you haven’t already done so, make sure to watch the introductory video for the Intermediate Chemistry and Advanced Chemistry lessons. They contain important information about the chemicals and lab equipment you’ll be using. When you’re done storing your chemicals out of reach, watch this video:
Please login or register to read the rest of this content.
Let’s see how much you’ve picked up with these experiments and the reading – answer as best as you can. (No peeking at the answers until you’re done!) Just relax and see what jumps to mind when you read the question. You can also print these out and jot down your answers in your science notebook.
Please login or register to read the rest of this content.
Let’s see how much you’ve picked up with these experiments and the reading – answer as best as you can. (No peeking at the answers until you’re done!) Just relax and see what jumps to mind when you read the question. You can also print these out and jot down your answers in your science notebook.
Please login or register to read the rest of this content.
Let’s see how you did! If you didn’t get a few of these, don’t let it stress you out – it just means you need to play with more experiments in this area. We’re all works in progress, and we have our entire lifetime to puzzle together the mysteries of the universe!
Here’s printer-friendly versions of the exercises and answers for you to print out: Simply click here for printable questions and answers.
Answers:
Please login or register to read the rest of this content.
Let’s see how you did! If you didn’t get a few of these, don’t let it stress you out – it just means you need to play with more experiments in this area. We’re all works in progress, and we have our entire lifetime to puzzle together the mysteries of the universe!
Here’s printer-friendly versions of the exercises and answers for you to print out: Simply click here for printable questions and answers.
Answers:
Please login or register to read the rest of this content.
You can go your whole life without paying any attention to the chemistry behind acids and bases. But you use acids and bases all the time! They are all around you. We identify acids and bases by measuring their pH.
Every liquid has a pH. If you pay particular attention to this lab, you will even be able to identify most acids and bases and understand why they do what they do. Acids range from very strong to very weak. The strongest acids will dissolve steel. The weakest acids are in your drink box. The strongest bases behave similarly. They can burn your skin or you can wash your hands with them.
Acid rain is one aspect of low pH that you can see every day if you look for it. This is a strange name, isn’t it? We get rained on all the time. If people were dissolving, if the rain made their skin smoke and burn, you’d think it would make headlines, wouldn’t you? The truth is acid rain is too weak to harm us except in very rare and localized conditions. But it’s hard on limestone buildings.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Purple and white colors, making the whitewash that Tom Sawyer used, and produce an exothermic chemical reaction…..does it get any better?
Limewater is one of the compounds we work with in this experiment. Limewater was used in the old days of America. We’re talking about the 80’s…..the 1880’s.
Traveling medicine shows sold what was called “patent medicines”. These usually had no medicinal properties at all. The man in charge, the salesman of the operation, was called a “huckster”. He would have the one of the people gathered around to listen to him blow into limewater. Their exhaled breath contains carbon dioxide, and the lime water turned cloudy, just like in our experiment.
The man would hold up the glass with the cloudy limewater in it and pour in some of his fantastic remedy. As long as the “medicine” was acidic, it would turn the cloudy limewater clear. This was proof that the remedy would cure whatever ailed the person.
Please login or register to read the rest of this content.
This experiment is for advanced students. This is a repeat of the experiment: Can Fish Drown? but now we’re going to do this experiment again with your new chemistry glassware.
The aquarium looked normal in every way, except for the fish. They were breathing very fast and sinking head first to the bottom of the tank. They would sink a few inches, then jerk into proper movement again.
The student had to figure out what was wrong. He had set up the aquarium as an ongoing science project, and it was his responsibility to maintain the fish tank. His grade depended on it.
He went to his mom for help. She looked over the setup. “Have you tested the water?”
A quizzical look on his face, the boy said, “Everything is normal nitrates, nitrites, hardness, alkalinity, and pH. The pH was a little acidic, but not outside the proper range.”
Please login or register to read the rest of this content.
This experiment is for advanced students.
Don’t put this in your car….yet. Hydrogen generation, capture, and combustion are big deals right now. The next phase of transportation, and a move away from fossil fuels in not found in electric cars. Electric cars are waiting until hydrogen fuel cell vehicles become practical. It can be done and is being done.
Cars being powered by hydrogen are here, but not on the market yet. Engineers and chemists are always finding new ways to improve the chemical reaction that produces hydrogen and making the vehicles more efficiently use the fuel. Hydrogen fuel is not just easy to make, it is inexpensive, and the “exhaust” is water.
We will generate hydrogen in this lab. We will also see how combustible it is. Just let your imagination wander….just a bit and you will see noiseless cars and trucks zipping along the streets and interstates, carrying people and cargo. The Indianapolis 500 wouldn’t be quite the same, though. “And there they go, roaring, I mean quietly entering turn two…”
Please login or register to read the rest of this content.
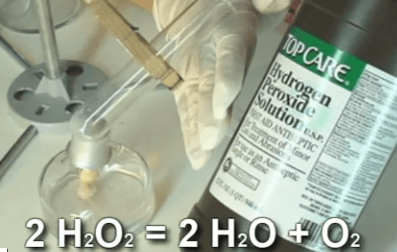
This experiment is for advanced students.
In industry, hydrogen peroxide is used in paper making to bleach the pulp before they form it into paper. Biologists, when preparing bones for display, use peroxide to whiten the bones.
At home, 3% peroxide combined with ammonium hydroxide is used to give dark-haired people their desired blonde moment. Peroxide is also used on wounds to clean them and remove dead tissue. Peroxide slows the flow from small blood vessels and oozing in wounds as well.
Please login or register to read the rest of this content.
This experiment is for advanced students.
This time we’re going to use a lot of equipment… really break out all the chemistry stuff. We’ll need all this stuff to generate oxygen with potassium permanganate (KMNO4). We will work with this toxic chemical and we will be careful…won’t we?
Please login or register to read the rest of this content.
This experiment is for advanced students.
Zinc (Zn), is a metal and it is found as element #30 on the periodic table. We need a little zinc to keep our bodies balanced, but too much is very dangerous.
Zinc is just like the common, everyday substance that we all know as di-hydrogen monoxide (which is the chemical name for water). We need water to survive, but too much will kill us.
DHMO: In chemistry, “Di” equals the number 2; hydrogen is H; mono equals the number one; and oxide is derived from oxygen, and its symbol is O. Put these together and you have Di-hydrogen (H2), and mono oxygen (O). Put them together, what do you have? Water!
Please login or register to read the rest of this content.
This experiment is for advanced students.
Lewis and Clark did this same experiment when they reached the Oregon coast in 1805. Men from the expedition traveled fifteen miles south of the fort they had built at the mouth of the Columbia River to where Seaside, Oregon now thrives.
In 1805, however, it was just men from the fort and Indians. They built an oven of rocks. For six weeks, they processed 1,400 gallons of seawater, boiling the water off to gain 28 gallons of salt.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Glo-sticks! Parents hang them from their trick or treaters, backpackers read with them light late at night in a tent. Glo-sticks work on the principle of chemiluminescence. Chemiluminescence is defined as emitting light without heat as the result of a chemical reaction.
Please login or register to read the rest of this content.
This experiment is for advanced students. This lab builds on concepts from the previous carbon dioxide lab.
Limewater….carbon dioxide…indicators. We don’t know too much about these things. Sure, we know a little. Carbon dioxide is exhaled by us and plants need it to grow. Burning fossil fuels produces carbon dioxide.
Indicators…something we observe that confirms to us that something specific is happening. Lime water turns cloudy and forms a precipitate in the presence of carbon dioxide. Blue litmus paper turns red in the presence of an acid. The dog barking at the door and dancing around indicates that you better let the dog out, and quick, to avoid….a pet spill?
Please login or register to read the rest of this content.
This experiment is for advanced students.
Who gets to burn something today? YOU get to burn something today!
You will be working with Zinc (Zn). Other labs in this kit allow us to burn metal, but there is a bit of a twist this time. We will be burning a powder.
Why a powder instead of a solid ribbon or foil as in the other labs? Have you heard of surface area being a factor in a chemical reaction? The more surface area there is to burn, the more dramatic the chemical change. So, with this fact in mind, a powder should burn faster or be more likely to burn than a large solid.
Please login or register to read the rest of this content.
Magnesium is one of the most common elements in the Earth’s crust. This alkaline earth metal is silvery white, and soft. As you perform this lab, think about why magnesium is used in emergency flares and fireworks. Farmers use it in fertilizers, pharmacists use it in laxatives and antacids, and engineers mix it with aluminum to create the BMW N52 6-cylinder magnesium engine block. Photographers used to use magnesium powder in the camera’s flash before xenon bulbs were available.
Most folks, however, equate magnesium with a burning white flame. Magnesium fires burn too hot to be extinguished using water, so most firefighters use sand or graphite.
We’re going to learn how to (safely) ignite a piece of magnesium in the first experiment, and next how to get energy from it by using it in a battery in the second experiment. Are you ready?
Please login or register to read the rest of this content.
This experiment is for advanced students.
Brimstone is another name for sulfur, and if you’ve ever smelled it burn…..whoa….I’m telling you ….you will see for yourself in this lab. It is quite a smell, for sure. Sulfur is element #6 on the periodic table. Sulfur is used in fertilizer, black powder, matches, and insecticides. In pioneer times sulfur was put into patent medicines and used as a laxative.
To further the evil reputation of sulfur, or brimstone, when sulfur is burned in a coal fired power plant, sulfur dioxide is produced. The sulfur is spewed into the air, where it is reacts with moisture in the air to form sulfuric acid. The clouds get full and need to let go of this sulfuric acid. Down comes the acid rain to wreak havoc on the masonry and plant life below.
Please login or register to read the rest of this content.
In this lab, we’re going to investigate the wonders of electrochemistry. Electrochemistry became a new branch of chemistry in 1832, founded by Michael Faraday. Michael Faraday is considered the “father of electrochemistry”. The knowledge gained from his work has filtered down to this lab. YOU will be like Michael Faraday. I imagined he would have been overjoyed to do this lab and see the results. You are soooo lucky to be able to take an active part in this experiment. Here’s what you’re going to do…
You will be “creating” metallic copper from a solution of copper sulfate and water, and depositing it on a negative electrode. Copper is one of our more interesting elements. Copper is a metal, and element 29 on your periodic table. It conducts heat and electricity very well.
Many things around you are made of copper. Copper wire is used in electrical wiring. It has been used for centuries in the form of pipes to distribute water and other fluids in homes and in industry. The Statue of Liberty is a wonderful example of how beautiful 180,000 pounds of copper can be. Yes, it is made of copper, and no, it doesn’t look like a penny…..on the surface. The green color is copper oxide, which forms on the surface of copper exposed to air and water. The oxide is formed on the surface and does not attack the bulk of the copper. You could say that copper oxide protects the copper.
Please login or register to read the rest of this content.
If we don’t have salt, we die. It’s that simple. The chemical formula for salt is NaCl. Broken down, we have Na (sodium) and Cl (chlorine). Either one of these can be fatal in sufficient quantities. When chemically combined, these two deadly elements become table salt. What once could kill now keeps us alive. Isn’t chemistry awesome?
Chlorine, element 17, is called a halogen as are all the elements in the 17th row. All halogens have similar chemical properties. They are highly reactive nonmetals, and react easily with most metals. Sodium is a metal, and is bonded with sodium in the table salt used in this lab. Besides being found in salt, chlorine has many uses in our world such as killing bacteria in our water, making plastic, cleaning products, and the list goes on. A very useful chemical, and is among the top ten chemicals produced in the United States. Ever since its discovery in 1774, chlorine has been very useful. It is found in nature in sodium chloride, but in very small concentrations. Seawater, the most abundant source of chlorine, has a concentration of only 19g of chlorine per liter.
Please login or register to read the rest of this content.
Electricity. Chemistry. Nothing in common, have nothing to do with each other. Wrong! Electrochemistry has been a fact since 1774. Once electricity was applied to particular solutions, changes occurred that scientists of the time did not expect.
In this lab, we will discover some of the same things that Farraday found over 300 years ago. We will be there as things tear apart, particles rush about, and the power of attraction is very strong. We’re not talking about dancing, we’re talking about something much more important and interesting….we’re talking about ELECTROCHEMISTRY!
Please login or register to read the rest of this content.
Ammonia has been used by doctors, farmers, chemists, alchemists, weightlifters, and our families since Roman times. Doctors revive unconscious patients, farmers use it in fertilizer, alchemists tried to use it to make gold, weightlifters sniff it into their lungs to invigorate their respiratory system and clear their heads prior to lifting tremendous loads. At home, ammonia is used to clean up the ketchup you spilled on the floor and never cleaned up.
The ammonia molecule (NH3) is a colorless gas with a strong odor – it’s the smell of freshly cleaned floors and windows. Mom is not cleaning with straight ammonia (it’s gas at room temperature because it boils at -28oF, so the stuff she cleans with is actually ammonium hydroxide, a solution of ammonia and water). Ammonia is found when plans and animals decompose, and it’s also in rainwater, volcanoes, your kidneys (to neutralize excess acid), in the ocean, some fertilizers, in Jupiter’s lower cloud decks, and trace amounts are found in our own atmosphere (it’s lighter than air).
Please login or register to read the rest of this content.
This experiment is for advanced students.
ACID!!! The word causes fear to creep in and get our attention.
BASIC!!! The word causes nothing to stir in most of us.
The truth is, a strong acid (pH 0-1) is dangerous, but a strong basic (pH 13-14) is just as dangerous. In this lab, we will get comfortable with the basics of bases and the acidity of acids along with how you can use both and tell the difference between them.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Ever use soap? Sodium hydroxide (NaOH) is the main component in lye soap. NaOH is mixed with some type of fat (vegetable, pig, cow, etc). Scent can be added for the ‘pretty’ factor and pumice or sand can be added for the manly “You’re coming off my hands and I’ll take no guff” factor. Lots of people still make their own soap and they enjoy doing it.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Potassium permanganate (KMnO4) in water turns an intense, deep, purple. It is important in the film industry for aging props and clothing to make them look much older than they are.
Also, artists use it in bone carving. People who carve antlers and bone use KMnO4 to darken the surface of the bone to make it look aged. They make the carving, soak it in potassium permanganate, then carve more, and repeat. The end result is a carving that has a light golden brown color. More dipping will darken the carving even more.
Potassium permanganate is going to undergo a chemical change with this activity. In this experiment, we will be able to witness several indicators of chemical change. Color changes, bubbles from gas generation, temperature change, and color disappearance are all indicators of chemical changes.
Please login or register to read the rest of this content.
This experiment is for advanced students.
How do you know if your brother is stealing your candy? Unwrap a wrapped hard candy that he likes a lot. Roll the candy around in the powdered food dye that matches the candy. (Push the powder into the candy so it “disappears”.) Re-wrap the candy. Set the candy in the place where it usually disappears from. Wait ten minutes after the candy disappears. Find your brother. He will be sporting a new color on his hands and mouth. Dye is hard to remove. It will have to be worn every day at school until it fades away as the skin cells slough off. The dye he now wears is in indicator that he has been taking your candy.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Sparks flying off in all directions…that’s fun. In this lab, we will show how easy it is to produce those shooting sparks. In a sparkler you buy at the store, the filings used are either iron or aluminum.
The filings are placed in a mixture that, when dry, adheres to the metal rod or stick that is used in making the sparkler. The different colors are created by adding different powdered chemicals to the mixture before it dries. When they burn, we get red, blue, white, and green.
Please login or register to read the rest of this content.
This experiment is for advanced students.
In gas form, element #59 is deadly. However, when iodine is in liquid form, it helps heal cuts and scrapes. The iodine molecule occurs in pairs, not as a single atom (many halogens do this, and it’s called a diatomic molecule). It’s hard to find iodine in nature, though it’s essential for staying healthy… too little iodine in the body takes a heavy toll on how well the brain operates.
A chunk of iodine is blackish-blue, and will sublimate (go from a solid straight to a gas, as seen in the photo here). Iodine is the heaviest element needed by living things. Iodized salt is sodium chloride fortified with iodine to prevent people from not getting enough iodine in their daily diets.
Iodine is found in seaweed (kelp) and seafood as well as vegetables that are grown in dirt that has high iodine levels. People that live inland and do not eat fish often have lower iodine levels than their coastal, fish-eating neighbors. The trick is not to get too much or too little iodine in your diet, because the symptoms of deficiency and excess levels are quite similar.
Starch (like cornstarch) are used as an indicator for detecting iodine in chemistry experiments. When combined with iodine, starch forms a blue-black color in the solution. We’re going to do this and many other activities in this lab, because this experiment is actually several labs rolled into one. First, we have to make iodine, store it, and then we get to use it in several experiments. Are you ready?
Please login or register to read the rest of this content.
This experiment is for advanced students.
Zinc and Hydrogen are important elements for all of us. Zinc (Zn) metal is element #30 on the periodic table. Lack of zinc in our diets will delay growth of our bodies and can kill.
Hydrogen gas (H) is element #1 on the periodic table. Hydrogen was discovered in the 1500s. In a pure state, hydrogen combustion (in small quantities) is interesting. In large amounts, mixed with oxygen, the explosion can be devastating.
Please login or register to read the rest of this content.
WARNING!! THIS EXPERIMENT IS PARTICULARLY DANGEROUS!! (No kidding.) This experiment is for advanced students.
We’ve created a video that shows you how to safely do this experiment, although if you’re nervous about doing this one, just watch the video and skip the actual experiment.
Bromine is a particularly nasty chemical, so be sure to very carefully follow the steps we’ve outlined in the video. You MUST do this experiment outdoors. We’ll be making a tiny amount to show how the chemical reactions involving bromine work.
Please login or register to read the rest of this content.
WARNING!! THIS EXPERIMENT IS PARTICULARLY DANGEROUS!! (No kidding.) This experiment is for advanced students.
We’ve created a video that shows you how to safely do this experiment, although if you’re nervous about doing this one, just watch the video and skip the actual experiment.
The gas you generate with this experiment is lethal in large doses, so you MUST do this experiment outdoors. We’ll be making a tiny amount to show how the chemical reactions of chlorine and hydrogen work.
Please login or register to read the rest of this content.
In this unit, you will learn how to build your own home chemistry lab safely under the direction of professionals. We’ll show you how to do real chemistry experiments, provide chemical storage information, give guidelines on proper chemical disposal when you’re finished, highlight lab tips and tricks, and warn you about things to watch out for. This is real chemistry for real kids.
This video picks up where the intermediate chemistry video leaves off so you’ll want to be sure you have completed that one first. The C3000 contains three trays, the first of which is the C1000 (which is covered in the intermediate chemistry lesson). So if you’ve completed the first part and are ready for more, here we go!
Please login or register to read the rest of this content.
In this unit, you will learn how to build your own home chemistry lab safely under the direction of professionals. We’ll show you how to do real chemistry experiments, provide chemical storage information, give guidelines on proper chemical disposal when you’re finished, highlight lab tips and tricks, and warn you about things to watch out for. This is real chemistry for real kids.
Please login or register to read the rest of this content.
Always have a FIRE EXTINGUISHER and ADULT HELP handy when performing fire experiments. NO EXCEPTIONS.
This video will show you how to transform the color of your flames. For a campfire, simply sprinkle the solids into your flames (make sure they are ground into a fine powder first) and you’ll see a color change. DO NOT do this experiment inside your house – the fumes given off by the chemicals are not something you want in your home!
One of the tricks to fire safety is to limit your fuel. The three elements you need for a flame are: oxygen, spark, and fuel. To extinguish your flames, you’ll have to either wait for the fuel to run out or smother the flames to cut off the oxygen. When you limit your fuel, you add an extra level of safety to your activities and a higher rate of success to your eyebrows.
Here’s what we’re going to do: first, make your spectrometer: you can make the simple spectrometer or the more-advanced calibrated spectrometer. Next, get your chemicals together and build your campfire. Finally, use your spectrometer to view your flames.
Please login or register to read the rest of this content.
Ever play with a prism? When sunlight strikes the prism, it gets split into a rainbow of colors. Prisms un-mix the light into its different wavelengths (which you see as different colors). Diffraction gratings are tiny prisms stacked together.
When light passes through a diffraction grating, it splits (diffracts) the light into several beams traveling at different directions. If you’ve ever seen the ‘iridescence’ of a soap bubble, an insect shell, or on a pearl, you’ve seen nature’s diffraction gratings.
Scientist use these things to split incoming light so they can figure out what fuels a distant star is burning. When hydrogen burns, it gives off light, but not in all the colors of the rainbow, only very specific colors in red and blue. It’s like hydrogen’s own personal fingerprint, or light signature.
While this spectrometer isn't powerful enough to split starlight, it's perfect for using with the lights in your house, and even with an outdoor campfire. Next time you're out on the town after dark, bring this with you to peek different types of lights - you'll be amazed how different they really are. You can use this spectrometer with your Colored Campfire Experiment also.
SPECIAL NOTE: This instrument is NOT for looking at the sun. Do NOT look directly at the sun. But you can point the tube at a sheet of paper that has the the sun’s reflected light on it.
Here's what you do:
This experiment is for advanced students.
One of my best teaching tools for science developed from a brain freeze one afternoon in class. I went to the board to draw the chlorophyll wheel and drew a complete blank.
“Let’s say I forgot how to draw the wheel.” I turned to the class, marker in hand, and scanned the room. Puzzled faces, the blank faces I expected, but, what was that? A few smiles scattered about the room.
As I pulled out and some volunteered info, we got into that wheel. They also found that it was easier to know what to do next than to have me tell them to find it in their book and be prepared…I was coming back to them. Students frantically finding the wheel in their biology books so they were armed when I came to them.
It was a great experience, and my lectures were a lot more fun and interactive from then on.
Please login or register to read the rest of this content.
This experiment is just for advanced students. If you guessed that this has to do with electricity and chemistry, you’re right! But you might wonder how they work together. Back in 1800, William Nicholson and Johann Ritter were the first ones to split water into hydrogen and oxygen using electrolysis. (Soon afterward, Ritter went on to figure out electroplating.) They added energy in the form of an electric current into a cup of water and captured the bubbles forming into two separate cups, one for hydrogen and other for oxygen.
This experiment is not an easy one, so feel free to skip it if you need to. You don’t need to do this to get the concepts of this lesson but it’s such a neat and classical experiment (my students love it) so you can give it a try if you want to. The reason I like this is because what you are really doing in this experiment is ripping molecules apart and then later crashing them back together.
Have fun and please follow the directions carefully. This could be dangerous if you’re not careful. The image shown here is using graphite from two pencils sharpened on both ends, but the instructions below use wire. Feel free to try both to see which types of electrodes provide the best results.