Getting Started
Requires Grades 9-12th Level Membership
Taught from a college-level, secular perspective, this course prepares students to engage with real science the way professional astronomers do.
Students will explore planets, stars, black holes, galaxies, and more through interactive labs, stargazing, real science projects, and weekly assignments that spark curiosity and build confidence.
Whether your student dreams of becoming a scientist or just loves looking at the night sky, this course is designed to ignite their passion for space and help them think like a real astronomer.
Each week, students will have a lesson with a teacher (watch live or recorded), build hands-on projects, and complete homework assignments. It's important that students complete each set of projects and assignments, as weeks will build on each other in complexity and content.
Near the end of the term, students will complete their capstone project. Students will have the opportunity to participate in scientific research that contributes to the real astronomical community—by measuring stars and submitting their findings for publication in a scientific journal.

How to Succeed in this Course
This is a true college-level astronomy course. That means we’ll be moving at the same pace, covering the same topics, and holding you to the same standards of work as you’d find in a university class. The material will challenge you, and success will require focus, persistence, and consistent effort.
But don’t worry—you won’t be left to figure it out on your own. We’ll walk you through the lessons step by step, provide guidance on assignments, and give you the support you need to succeed. What we can’t do, however, is do the work for you. To get the most out of this course, you’ll need to show up prepared, participate fully, and commit to completing each assignment and project on time.
If you put in the effort, you’ll not only build a strong foundation in astronomy, but you’ll also experience the excitement of doing real science—discovering, questioning, and thinking like a professional astronomer.
Special Concern: How Can Faith-Based Homeschool Students Succeed in Secular College Science Courses?
How do astronomers find planets around distant stars? If you look at a star through binoculars or a telescope, you’ll quickly notice how bright the star is, and how difficult it is to see anything other than the star, especially a small planet that doesn’t generate any light of its own! Astronomers look for a shift, or wobble, of the star as it gets gravitationally “yanked” around by the orbiting planets. By measuring this wobble, astronomers can estimate the size and distance of larger orbiting objects.
Doppler spectroscopy is one way astronomers find planets around distant stars. If you recall the lesson where we created our own solar system in a computer simulation, you remember how the star could be influenced by a smaller planet enough to have a tiny orbit of its own. This tiny orbit is what astronomers are trying to detect with this method.
Materials
- Several bouncy balls of different sizes and weights, soft enough to stab with a toothpick
- Toothpicks
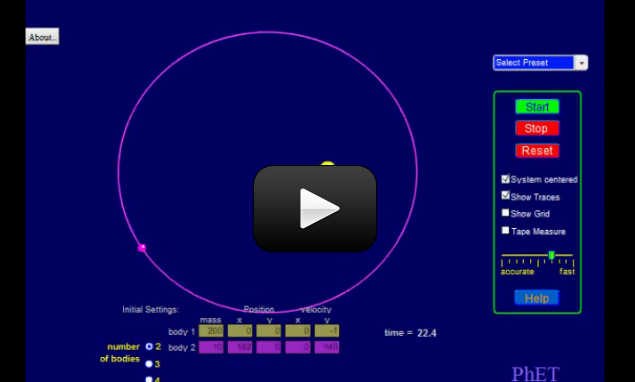
 What would happen if our solar system had three suns? Or the Earth had two moons? You can find out all these and more with this lesson on orbital mechanics. Instead of waiting until you hit college, we thought we’d throw some university-level physics at you… without the hard math.
What would happen if our solar system had three suns? Or the Earth had two moons? You can find out all these and more with this lesson on orbital mechanics. Instead of waiting until you hit college, we thought we’d throw some university-level physics at you… without the hard math.
Please login or register to read the rest of this content.
Ever wonder exactly how far away the planets really are? Here’s the reason they usually don’t how the planets and their orbits to scale – they would need a sheet of paper nearly a mile long!
To really get the hang of how big and far away celestial objects really are, find a long stretch of road that you can mark off with chalk. We’ve provided approximate (average) orbital distances and sizes for building your own scale model of the solar system.
When building this model, start by marking off the location of the sun (you can use chalk or place the objects we have suggested below as placeholders for the locations). Are you ready to find out what’s out there? Then let’s get started.
Materials:
- measuring tape (the biggest one you have)
- tape or chalk to mark off the locations
- 2 grains of sand or white sugar
- 12″ beach ball
- 3 peppercorns
- golf or ping pong ball
- shooter-size marble
- 2 regular-size marbles
Please login or register to read the rest of this content.
Please login or register to read the rest of this content.
One common misconception is that the seasons are caused by how close the Earth is to the Sun. Today you get to do an experiment that shows how seasons are affected by axis tilt, not by distance from the Sun. And you also find out which planet doesn’t have sunlight for 42 years.
The seasons are caused by the Earth’s axis tilt of 23.4o from the ecliptic plane.
Materials
- Bright light source (not fluorescent)
- Balloon
- Protractor
- Masking tape
- 2 liquid crystal thermometers
- Ruler, yardstick or meter stick
- Marker
If the cell has a nucleus, the DNA is located in the nucleus. If not, it is found in the cytoplasm. DNA is the genetic material that has all the information about a cell.
DNA is a long molecule found in the formed by of two strands of genes. DNA carries two copies—two “alleles”—of each gene. Those alleles can either be similar to each other (homozygous), or dissimilar (heterozygous).
We’re going to learn how to extract DNA from any fruit or vegetable you have lying around the fridge. Are you ready?
Today you get to learn how to read an astronomical chart to find out when the Sun sets, when twilight ends, which planets are visible, when the next full moon occurs, and much more. This is an excellent way to impress your friends.
The patterns of stars and planets stay the same, although they appear to move across the sky nightly, and different stars and planets can be seen in different seasons.
Materials:
- Printout of Stargazer’s Almanac
- Pencil
- Tape and scissors (optional)
- Ruler
 This experiment is one of my favorites in this acceleration series, because it clearly shows you what acceleration looks like.
This experiment is one of my favorites in this acceleration series, because it clearly shows you what acceleration looks like.
The materials you need is are:
- a hard, smooth ball (a golf ball, racket ball, pool ball, soccer ball, etc.)
- tape or chalk
- a slightly sloping driveway (you can also use a board for a ramp that’s propped up on one end)
For advanced students, you will also need: a timer or stopwatch, pencil, paper, measuring tape or yard stick, and this printout.
Grab a friend to help you out with this experiment – it’s a lot easier with two people.
Are you ready to get started really discovering what acceleration is all about?
Here’s what you do:
Please login or register to read the rest of this content.
Please login or register to read the rest of this content.
Newton’s Second Law is one of the toughest of the laws to understand but it is very powerful. In its mathematical form, it is so simple, it’s elegant. Mathematically it is F=ma or Force = Mass x Acceleration. An easy way to remember that is to think of your mother trying to get you out of bed in the morning. Force equals MA’s coming to get you! (I did mention how bad physics jokes are, right?)
Measuring Photosynthesis of a Leaf
All organisms, from tigers to tulips, need energy. Even bacteria need energy. To get this energy, organisms access stored energy by eating food. Cows eat grass. Lions eat cows. But if you look closely at it, all the food energy actually can be traced back to the light from the sun.
All energy we have available on earth actually comes from the sun. When sun falls on the trees and grass, they use it to make chemical energy it can use later for things it needs. That energy is transferred to the animals that eat the plants.
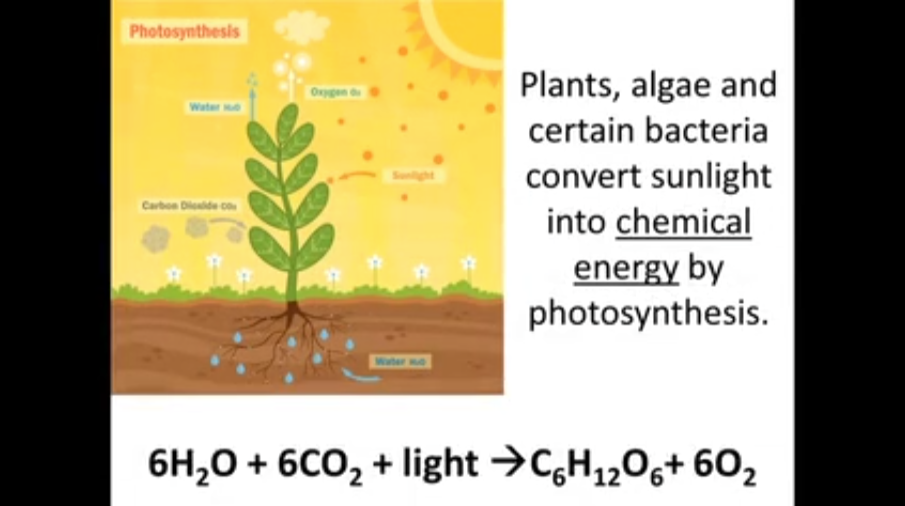
The main part of the oxygen cycle on earth is photosynthesis, which converts sunlight into energy for the plant and oxygen for our atmosphere. Plants, green algae and a number of micro-organisms take light, six molecules of water, six molecules of carbon dioxide to make one sugar molecule and six oxygen molecules.
For plants, carbon dioxide enters through the breathing pores on the surface of a plant's leaves (called the stomata), and the water and nutrients enter through the roots. The oxygen gas leaves the leaf through the stomata pores and the sugar (glucose) is distributed to the rest of the plant.
We’re going to measure the rate of photosynthesis of a plant. You basically take small bits of a leaf like spinach, stick it in a cup of water that has extra carbon dioxide in it, and shine a light on it. The plant will take the carbon dioxide from the water and the light from the lamp and make oxygen bubbles that stick to it and lift it to the surface of the water, like a kid holding a bunch of helium balloons. And you time how long this all takes and you have the rate of photosynthesis for your leaf.
Once you’ve got this experiment working, think about other things that might affect the rate of photosynthesis. What about the color of the light (is red better, or yellow, blue, green, UV…?) Does it matter how far the light is from the heat sink? Does the type of leaf matter?
Lab Time:
- Cut out small samples using hole punch
- Place leaves in a cup of water (or use a water-filled syringe)
- Get the leaves to sink by pressing out all air bubbles (plunge syringe several times).
- Add a tsp of a carbon source (like baking soda)
- Place a heat sink on top of the first cup (like another clear cup of water)
- Place in direct sunlight or under a lamp
- Your leave “chads” should rise to the surface if they are generating enough oxygen bubbles!
- Try different leaves, add more/less carbon, change water temp… and have fun!
Additional Notes for the Lab:
Make sure you don’t shine your light directly on the leaf or the glass of water it’s in, or you’ll be adding heat, not just light, to your experiment. Use a clear glass for the main cup of water, and then put a second glass of water on top, and shine your light through the top glass into the lower glass. We don’t want to heat up the water with the leaves because that will change our experiment.
The leaf will absorb energy from the light and convert H2O to make oxygen bubbles. When enough oxygen bubbles are attached to the leaf, the leaf floats to the top and you can time how long it takes that leave to float to the drop from when you first drop it in and switch on the light.
Also, not every kind of leaf is going to work for this experiment. My favorite leaf to use is spinach, but again, go ahead and try different varieties. You know you’ve done it right when the leaves fall to the bottom of the syringe. You can also try pressing the leaf underwater between your thumb and the side of the glass, or leaving it in a dark cupboard overnight to soak in the water. If it still doesn’t sink, discard it and try a different kind of leaf.
Exercises:
1. Plants, algae and certain bacteria convert sunlight _______________________ into by photosynthesis.
2. Write out what each one of these means in plain everyday words:
6 H2O + 6 CO2 + light --> C6H12O6 + 6 O2

How does the eye work? If you are amazed as I am about how the different parts of the eye are put together, then this is the lab for you! It's important not only to learn how to take apart video cameras and blenders to find out how they work, but also to be fascinated by how the different parts of living creatures work ... like the eye!
In today’s dissection, we’ll be looking at a cow eye. Because cow eyes are so similar to humans eyes, you’ll learn a lot about your own eyes by dissecting the cow eye. Eyes are a very special organ that form images from the world around you and then send the images to your brain for processing. You will be able to see the cornea, iris, pupil, connecting muscles and veins, and other features.
Materials:
Please login or register to read the rest of this content.
Click here to go to part:28 Finale!
Dissection in biology provides a hands-on education above and beyond reading a textbook. By seeing, touching and exploring different organs, muscles and tissues inside an animal and seeing how they work together allows you to really understand your own body and appreciate the amazing world around us. And it's not hard - you can dissect a kidney right at home using an inexpensive specimen with a dissection guide and simple dissection tools! Many doctors, surgeons and veterinarians report that their first fascination with the body started with a biology dissection class.
In today’s dissection, we’ll be looking at a kidney. Kidneys are critical for removing toxic waste and regulating the levels of water, sugars, salts, and acids in the bodies of mammals. There are many things that make a kidney interesting, including its unique bean shape and the fact that it contains about a million microscopic structures called nephrons that are key in the blood filtration process.
Materials:
Please login or register to read the rest of this content.
Click here to go to part 27:Cow Eye Dissection
Dissection in biology provides a hands-on education above and beyond reading a textbook. By seeing, touching and exploring different organs, muscles and tissues inside an animal and seeing how they work together allows you to really understand your own body and appreciate the amazing world around us. And it's not hard - you can dissect a sheep brain right at home using an inexpensive specimen with a dissection guide and simple dissection tools! Many doctors, surgeons and veterinarians report that their first fascination with the body started with a biology dissection class.
In today’s dissection, we’ll be looking at a sheep brain. Brains, while still not entirely understood by biologists or psychologists, are critical for movement, respiration, thought, memory, processing sensory signals, and more. What we talk about in today’s dissection just scratches the surface of all there is to know about the brain, which is the most complex organ in the human body.
Materials:
Please login or register to read the rest of this content.
Click here to go to part:26 Sheep Kidney Dissection
Dissection in biology provides a hands-on education above and beyond reading a textbook. By seeing, touching and exploring different organs, muscles and tissues inside an animal and seeing how they work together allows you to really understand your own body and appreciate the amazing world around us. And it's not hard - you can dissect a heart right at home using an inexpensive specimen with a dissection guide and simple dissection tools! Many doctors, surgeons and veterinarians report that their first fascination with the body started with a biology dissection class.
In today’s dissection, we’ll be looking at a sheep heart. Like humans, sheep have four-chambered hearts. Hearts are an essential organ--they pump blood through your body to keep you alive!
Materials:
Please login or register to read the rest of this content.
Click here to go to part:25 Sheep Brain Dissection
In today’s dissection, we’ll be looking at an owl pellet. Owls are carnivores, and they eat things like moles, shrews, rodents, birds, insects, and even crayfish. Owls are unable to digest the bones and fur of these creatures, so they regurgitate (or spit up) what are called pellets--small bundles of all the indigestible parts of the owl’s prey.
Owl pellet dissection is an easy, hands-on way to learn about the eating habits of birds of prey. (Owl pellets are the regurgitated remains of an owl's meal.) But don't be grossed out - finding and piecing together the bones inside owl pellets is fascinating work for a young scientist such as yourself! As you dissect the pellet, you'll find skeletons of mice, voles, birds, and more. Synthetic pellets are available for younger children if you'd like to use a substitute.
Dissection in biology provides a hands-on education above and beyond reading a textbook. By seeing, touching and exploring different organs, muscles and tissues inside an animal and seeing how they work together allows you to really understand your own body and appreciate the amazing world around us. And it's not hard - you can dissect a pellet right at home using an inexpensive specimen with a dissection guide and simple dissection tools! Many doctors, surgeons and veterinarians report that their first fascination with the body started with a biology dissection class.
Materials:
Please login or register to read the rest of this content.
Click here to go to part:24 Sheep Heart Dissection
Dissection in biology provides a hands-on education above and beyond reading a textbook. By seeing, touching and exploring different organs, muscles and tissues inside an animal and seeing how they work together allows you to really understand your own body and appreciate the amazing world around us. And it’s not hard – you can dissect a frog right at home using an inexpensive specimen with a dissection guide and simple dissection tools! Many doctors, surgeons and veterinarians report that their first fascination with the body started with a biology dissection class.
In today’s dissection, we’ll be looking at a frog. Frogs are members of the Class Amphibia. There are many things that make frogs interesting: they live both in water and on land, they actually begin life in water as limbless tadpoles, and some can change color depending on their environment.
Materials
- Frog (click if you need the whole frog kit, or here if you need only the frog)
- Dissection tools
- Dissection pins
- Dissection guide
Please login or register to read the rest of this content.
Click here to go to part:23 Owl Pellet Dissection
Dissection in biology provides a hands-on education above and beyond reading a textbook. By seeing, touching and exploring different organs, muscles and tissues inside an animal and seeing how they work together allows you to really understand your own body and appreciate the amazing world around us. And it’s not hard – you can dissect a crayfish right at home using an inexpensive specimen with a dissection guide and simple dissection tools! Many doctors, surgeons and veterinarians report that their first fascination with the body started with a biology dissection class.
In today’s dissection, we’ll be looking at a crayfish. Crayfish are members of the phylum Arthropoda. There are many things that make crayfish interesting: they dwell at the bottom of streams, rivers, and ponds; they feed on just about anything that comes their way (that’s why they’re called freshwater scavengers); and they have many appendages that help them save energy.
Materials
- Crayfish (click if you need the whole kit, or just the crayfish)
- Dissection tools
- Dissection guide
Please login or register to read the rest of this content.
Click here to go to part 22:Dissecting a Frog
Dissection in biology provides a hands-on education above and beyond reading a textbook. By seeing, touching and exploring different organs, muscles and tissues inside an animal and seeing how they work together allows you to really understand your own body and appreciate the amazing world around us. And it’s not hard – you can dissect a starfish right at home using an inexpensive specimen with a dissection guide and simple dissection tools! Many doctors, surgeons and veterinarians report that their first fascination with the body started with a biology dissection class.
In today’s dissection, we’ll be looking at a starfish. Starfish are members of the phylum Echinoderm. There are many things that make starfish interesting: their rays are symmetrical around their center (this is called radial symmetry), they use seawater instead of blood to transport nutrients through their bodies (this is called a water vascular system), and they move around using tube feet on the underside of their bodies.
Materials
- Starfish (click if you need the whole kit, or just the starfish)
- Dissection tools
- Dissection guide
Please login or register to read the rest of this content.
Click here to go to part:21 Dissecting a Crayfish
Dissection in biology provides a hands-on education above and beyond reading a textbook. By seeing, touching and exploring different organs, muscles and tissues inside an animal and seeing how they work together allows you to really understand your own body and appreciate the amazing world around us. And it's not hard - you can dissect a fish right at home using an inexpensive specimen with a dissection guide and simple dissection tools! Many doctors, surgeons and veterinarians report that their first fascination with the body started with a biology dissection class
.
In today’s dissection, we’ll be looking at a perch. Perch are members of the phylum Chordata. There are many things that make perch interesting: they are bony fishes which make them “true” fishes, they live in both freshwater and saltwater, and their diets change based on how big they are.
Materials:
Please login or register to read the rest of this content.
Click here to go to part 20:Dissecting a Starfish
Dissection in biology provides a hands-on education above and beyond reading a textbook. By seeing, touching and exploring different organs, muscles and tissues inside an animal and seeing how they work together allows you to really understand your own body and appreciate the amazing world around us. And it's not hard - you can dissect a grasshopper right at home using this inexpensive specimen with a dissection guide and simple dissection tools! Many doctors, surgeons and veterinarians report that their first fascination with the body started with a biology dissection class.
Materials:
- Grasshopper (click if you need the whole kit or just the grasshopper)
- Dissection tools
- Dissection Guide
Please login or register to read the rest of this content.
Click here to go to part:19 Perch Dissection
Dissection in biology provides a hands-on education above and beyond reading a textbook. By seeing, touching and exploring different organs, muscles and tissues inside an animal and seeing how they work together allows you to really understand your own body and appreciate the amazing world around us. And it's not hard - you can dissect a worm right at home using an inexpensive specimen with a dissection guide and simple dissection tools! Many doctors, surgeons and veterinarians report that their first fascination with the body started with a biology dissection class
.
In today’s dissection, we’ll be looking at an earthworm. Earthworms play an important role in their ecosystem--when they tunnel through dirt they mix nutrients which helps make the soil healthy and able to support plant life.
Materials:
Please login or register to read the rest of this content.
Click here to go to part:18 Grasshopper Dissection
This experiment is just for advanced students. If you guessed that this has to do with electricity and chemistry, you’re right! But you might wonder how they work together. Back in 1800, William Nicholson and Johann Ritter were the first ones to split water into hydrogen and oxygen using electrolysis. (Soon afterward, Ritter went on to figure out electroplating.) They added energy in the form of an electric current into a cup of water and captured the bubbles forming into two separate cups, one for hydrogen and other for oxygen.
This experiment is not an easy one, so feel free to skip it if you need to. You don’t need to do this to get the concepts of this lesson but it’s such a neat and classical experiment (my students love it) so you can give it a try if you want to. The reason I like this is because what you are really doing in this experiment is ripping molecules apart and then later crashing them back together.
Have fun and please follow the directions carefully. This could be dangerous if you’re not careful. The image shown here is using graphite from two pencils sharpened on both ends, but the instructions below use wire. Feel free to try both to see which types of electrodes provide the best results.
Please login or register to read the rest of this content.
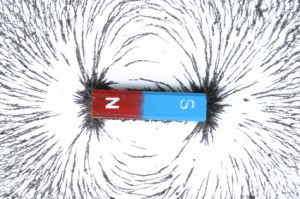
Magnetic fields are created by electrons moving in the same direction. A magnetic field must come from a north pole of a magnet and go to a south pole of a magnet (or atoms that have turned to the magnetic field.) Iron and a few other types of atoms will turn to align themselves with the magnetic field. Compasses turn with the force of the magnetic field.
If an object is filled with atoms that have an abundance of electrons spinning in the same direction, and if those atoms are lined up in the same direction, that object will have a magnetic force.
Materials: magnet wire, nail, magnet, compass, 12VDC motor, bi-polar LED, D-cell battery, sandpaper Please login or register to read the rest of this content.
Materials: balloon, magnet, small objects like paper clips or iron filings
Please login or register to read the rest of this content.
Materials: magnet you can break or cut in half, scissors or hammer (depending on the size of your magnet)
Please login or register to read the rest of this content.
Materials: balloon
Please login or register to read the rest of this content.
Force is a push or a pull, like pulling a wagon or pushing a car. Gravity is a force that attracts things to one another. Weight is a measure of how much gravity is pulling on an object.
Gravity accelerates all things equally. Which means all things speed up (accelerate) the same amount as they fall. Acceleration is the rate of change in velocity. In other words, how fast is a change in speed and/or a change in direction happening.
Materials: balloon Please login or register to read the rest of this content.
Materials: garden hose connected to a water faucet
Please login or register to read the rest of this content.
Materials: ball
Please login or register to read the rest of this content.
This is a nit-picky experiment that focuses on the energy transfer of rolling cars. You’ll be placing objects and moving them about to gather information about the potential and kinetic energy.
We’ll also be taking data and recording the results as well as doing a few math calculations, so if math isn’t your thing, feel free to skip it.
Here’s what you need:
We’re going to build monster roller coasters in your house using just a couple of simple materials. You might have heard how energy cannot be created or destroyed, but it can be transferred or transformed (if you haven’t that’s okay – you’ll pick it up while doing this activity).
Roller coasters are a prime example of energy transfer: You start at the top of a big hill at low speeds (high gravitational potential energy), then race down a slope at break-neck speed (potential transforming into kinetic) until you bottom out and enter a loop (highest kinetic energy, lowest potential energy). At the top of the loop, your speed slows (increasing your potential energy), but then you speed up again and you zoom near the bottom exit of the loop (increasing your kinetic energy), and you’re off again!
Here’s what you need:
Bobsleds use the low-friction surface of ice to coast downhill at ridiculous speeds. You start at the top of a high hill (with loads of potential energy) then slide down a icy hill til you transform all that potential energy into kinetic energy. It’s one of the most efficient ways of energy transformation on planet Earth. Ready to give it a try?
This is one of those quick-yet-highly-satisfying activities which utilizes ordinary materials and turns it into something highly unusual… for example, taking aluminum foil and marbles and making it into a racecar.
While you can make a tube out of gift wrap tubes, it’s much more fun to use clear plastic tubes (such as the ones that protect the long overhead fluorescent lights). Find the longest ones you can at your local hardware store. In a pinch, you can slit the gift wrap tubes in half lengthwise and tape either the lengths together for a longer run or side-by-side for multiple tracks for races. (Poke a skewer through the rolls horizontally to make a quick-release gate.)
Here’s what you need:
- aluminum foil
- marbles (at least four the same size)
- long tube (gift wrapping tube or the clear protective tube that covers fluorescent lighting is great)
This experiment is for Advanced Students.There are several different ways of throwing objects. This is the only potato cannon we’ve found that does NOT use explosives, so you can be assured your kid will still have their face attached at the end of the day. (We’ll do more when we get to chemistry, so don’t worry!)
These nifty devices give off a satisfying *POP!!* when they fire and your backyard will look like an invasion of aliens from the French Fry planet when you’re done. Have your kids use a set of goggles and do all your experimenting outside.
Here’s what you need:
When you drop a ball, it falls 16 feet the first second you release it. If you throw the ball horizontally, it will also fall 16 feet in the first second, even though it is moving horizontally… it moves both away from you and down toward the ground. Think about a bullet shot horizontally. It travels a lot faster than you can throw (about 2,000 feet each second). But it will still fall 16 feet during that first second. Gravity pulls on all objects (like the ball and the bullet) the same way, no matter how fast they go.
What if you shoot the bullet faster and faster? Gravity will still pull it down 16 feet during the first second, but remember that the surface of the Earth is round. Can you imagine how fast we’d need to shoot the bullet so that when the bullet falls 16 feet in one second, the Earth curves away from the bullet at the same rate of 16 feet each second?
Answer: that bullet needs to travel nearly 5 miles per second. (This is also how satellites stay in orbit – going just fast enough to keep from falling inward and not too fast that they fly out of orbit.)
Catapults are a nifty way to fire things both vertically and horizontally, so you can get a better feel for how objects fly through the air. Notice when you launch how the balls always fall at the same rate – about 16 feet in the first second. What about the energy involved?
When you fire a ball through the air, it moves both vertically and horizontally (up and out). When you toss it upwards, you store the (moving) kinetic energy as potential energy, which transfers back to kinetic when it comes whizzing back down. If you throw it only outwards, the energy is completely lost due to friction.
The higher you pitch a ball upwards, the more energy you store in it. Instead of breaking our arms trying to toss balls into the air, let’s make a simple machine that will do it for us. This catapult uses elastic kinetic energy stored in the rubber band to launch the ball skyward.
This is a very simple yet powerful demonstration that shows how potential energy and kinetic energy transfer from one to the other and back again, over and over. Once you wrap your head around this concept, you’ll be well on your way to designing world-class roller coasters.
For these experiments, find your materials:
- some string
- a bit of tape
- a washer or a weight of some kind
- set of magnets (at least 6, but more is better)
When you toss down a ball, gravity pulls on the ball as it falls (creating kinetic energy) until it smacks the pavement, converting it back to potential energy as it bounces up again. This cycles between kinetic and potential energy as long as the ball continues to bounce.
Please login or register to read the rest of this content.
We’re going to experiment with Newton’s Third law by blowing up balloons and letting them rocket, race, and zoom all over the place. When you first blow up a balloon, you’re pressurizing the inside of the balloon by adding more air (from your lungs) into the balloon. Because the balloon is made of stretchy rubber (like a rubber band), the balloon wants to snap back into the smallest shape possible as soon as it gets the chance (which usually happens when the air escapes through the nozzle area). And you know what happens next – the air inside the balloon flows in one direction while the balloon zips off in the other.
Question: why does the balloon race all over the room? The answer is because of something called ‘thrust vectoring’, which means you can change the course of the balloon by angling the nozzle around. Think of the kick you’d feel if you tried to angle around a fire hose operating at full blast. That kick is what propels balloons and fighter aircraft into their aerobatic tricks.
We’re going to perform several experiments here, each time watching what’s happening so you get the feel for the Third Law. You will need to find:
- balloons
- string
- wood skewer
- two straws
- four caps (like the tops of milk jugs, film canisters, or anything else round and plastic about the size of a quarter)
- wooden clothespin
- a piece of stiff cardboard (or four popsicle sticks)
- hot glue gun
First, let’s experiment with the balloon. Here’s what you can do:
A common misconception in science is that centrifugal and centripetal force (or acceleration) are the same thing. These two terms constantly throw students into frenzy, mostly because there is no clear definition in most textbooks. Here’s the scoop: centripetal and centrifugal force are NOT the same thing!
This experiment is mostly for Advanced Students, but here’s a quick lesson you can do with your younger students…
Please login or register to read the rest of this content.
Please login or register to read the rest of this content.
Newton’s Second Law is one of the toughest of the laws to understand but it is very powerful. In its mathematical form, it is so simple, it’s elegant. Mathematically it is F=ma or Force = Mass x Acceleration. An easy way to remember that is to think of your mother trying to get you out of bed in the morning. Force equals MA’s coming to get you! (I did mention how bad physics jokes are, right?)
 Gyroscopes defy human intuition, common sense, and even appear to defy gravity. You’ll find them in aircraft navigation instruments, games of Ultimate Frisbee, fast bicycles, street motorcycles, toy yo-yos, and the Hubble Space Telescope. And of course, the toy gyroscope (as shown here). Gyroscopes are used at the university level to demonstrate the principles of angular momentum, which is what we’re going to learn about here.
Gyroscopes defy human intuition, common sense, and even appear to defy gravity. You’ll find them in aircraft navigation instruments, games of Ultimate Frisbee, fast bicycles, street motorcycles, toy yo-yos, and the Hubble Space Telescope. And of course, the toy gyroscope (as shown here). Gyroscopes are used at the university level to demonstrate the principles of angular momentum, which is what we’re going to learn about here.
If you happen to have one of these toy gyroscopes, pull it out and play with it (although it’s not essential to this experiment). Notice that you can do all sorts of things with it when you spin it up, such as balance it on one finger (or even on a tight string). Wrap one end with string and hold the string vertically and you’ll find the gyro slowly rotates about the vertical string instead of flopping downward (as most objects do in Earth’s gravitational field). But why? Here’s the answer in plain English:
Please login or register to read the rest of this content.
Please login or register to read the rest of this content.
 This experiment is one of my favorites in this acceleration series, because it clearly shows you what acceleration looks like.
This experiment is one of my favorites in this acceleration series, because it clearly shows you what acceleration looks like.
The materials you need is are:
- a hard, smooth ball (a golf ball, racket ball, pool ball, soccer ball, etc.)
- tape or chalk
- a slightly sloping driveway (you can also use a board for a ramp that’s propped up on one end)
For advanced students, you will also need: a timer or stopwatch, pencil, paper, measuring tape or yard stick, and this printout.
Grab a friend to help you out with this experiment – it’s a lot easier with two people.
Are you ready to get started really discovering what acceleration is all about?
Here’s what you do:
Please login or register to read the rest of this content.
Please login or register to read the rest of this content.
Velocity is always tangent to the circle in the direction of the motion, and acceleration is always directed radially inward. Because of these two things, the acceleration that arises from traveling in a circle is called centripetal acceleration (a word created by Sir Isaac Newton). There’s no direct relationship between the acceleration and velocity vectors for a moving particle.
Please login or register to read the rest of this content.
If you jump out of an airplane, how fast would you fall? What’s the greatest speed you would reach? Let’s practice figuring it out without jumping out of a plane.
This experiment will help you get the concept of velocity by allowing you to measure the rate of fall of several objects. It’s also a great experiment to record in your science journal.
First, you’ll need to find your materials:
Ever wonder how magicians work their magic? This experiment is worthy of the stage with a little bit of practice on your end.
Here’s how this activity is laid out: First, watch the video below. Next, try it on your own. Make sure to send us your photos of your inventions here!
Next time you watch a drag race, notice the wheels. Are they solid metal discs, or do they have holes drilled through the rims? I came up with this somewhat silly, but incredibly powerful quick science demonstration to show my 2nd year university students how one set of rims could really make a difference on the racetrack (with all other things being equal).
Please login or register to read the rest of this content.
Please login or register to read the rest of this content.
This lesson may give you a sinking sensation but don’t worry about it. It’s only because we’re talking about gravity. You can’t go anywhere without gravity. Even though we deal with gravity on a constant basis, there are several misconceptions about it. Let’s get to an experiment right away and I’ll show you what I mean.
If I drop a ping pong ball and a golf ball from the same height, which one hits the ground first? How about a bowling ball and a marble?
Here’s what you need:
It is very rare, especially on Earth, to have an object that is experiencing force from only one direction. A bicycle rider has the force of air friction pushing against him. He has to fight against the friction between the gears and the wheels. He has gravity pulling down on him. His muscles are pushing and pulling inside him and so on and so on.
Even as you sit there, you have at least two forces pushing and pulling on you. The force of gravity is pulling you to the center of the Earth. The chair is pushing up on you so you don’t go to the center of the Earth. So with all these forces pushing and pulling, how do you keep track of them all? That’s where net force comes in.
The net force is when you add up all of the forces on something and see what direction the overall force pushes in. The word “net”, in this case, is like net worth or net income. It’s a mathematical concept of what is left after everything that applies is added and subtracted. The next activity will make this clearer.
Here’s what you need:
 Did you know that your cereal may be magnetic? Depending on the brand of cereal you enjoy in the morning, you’ll be able to see the magnetic effects right in your bowl. You don’t have to eat this experiment when you’re done, but you may if you want to (this is one of the ONLY times I’m going to allow you do eat what you experiment with!) For a variation, pull out all the different boxes of cereal in your cupboard and see which has the greatest magnetic attraction.
Did you know that your cereal may be magnetic? Depending on the brand of cereal you enjoy in the morning, you’ll be able to see the magnetic effects right in your bowl. You don’t have to eat this experiment when you’re done, but you may if you want to (this is one of the ONLY times I’m going to allow you do eat what you experiment with!) For a variation, pull out all the different boxes of cereal in your cupboard and see which has the greatest magnetic attraction.
Have you ever been close to something that smells bad? Have you noticed that the farther you get from that something, the less it smells, and the closer you get, the more it smells? Well forces sort of work in the same way.
Forces behave according to a fancy law called the inverse-square law. To be technical, an inverse-square law is any physical law stating that some physical quantity or strength is inversely proportional to the square of the distance from the source of that physical quantity.
The inverse-square law applies to quite a few phenomena in physics. When it comes to forces, it basically means that the closer an object comes to the source of a force, the stronger that force will be on that object. The farther that same object gets from the force’s source, the weaker the effect of the force.
Mathematically we can say that doubling the distance between the object and the source of the force makes the force 1/4th as strong. Tripling the distance makes the force 1/9th as strong. Let’s play with this idea a bit.
Here’s what you need:
 You are actually fairly familiar with electric fields too, but you may not know it. Have you ever rubbed your feet against the floor and then shocked your brother or sister? Have you ever zipped down a plastic slide and noticed that your hair is sticking straight up when you get to the bottom? Both phenomena are caused by electric fields and they are everywhere!
You are actually fairly familiar with electric fields too, but you may not know it. Have you ever rubbed your feet against the floor and then shocked your brother or sister? Have you ever zipped down a plastic slide and noticed that your hair is sticking straight up when you get to the bottom? Both phenomena are caused by electric fields and they are everywhere!
 The electromagnetic field is a bit strange. It is caused by either a magnetic field or an electric field moving. If a magnetic field moves, it creates an electric field. If an electric field moves, it creates a magnetic field.
The electromagnetic field is a bit strange. It is caused by either a magnetic field or an electric field moving. If a magnetic field moves, it creates an electric field. If an electric field moves, it creates a magnetic field.
Please login or register to read the rest of this content.
Please login or register to read the rest of this content.
Remember, there are four different kinds of forces: strong nuclear force,
electromagnetism, weak nuclear force, and gravity. There are also four basic force fields that you come into contact with all the time. They are the gravitational field, the electric field, the magnetic field, and the electromagnetic field. Notice that those four force fields really only use two of the four different kinds of force: electromagnetism and gravity. Let’s take a quick look at what causes these four fields and what kind of objects they can affect, starting with the magnetic field.
Here’s what you need:
Please login or register to read the rest of this content.
Ok, sort of a silly experiment I admit. But here’s what we’re going for – there is an invisible force acting on you and the ball. As you will see in later lessons, things don’t change the way they are moving unless a force acts on them. When you jump, the force that we call gravity pulled you back to Earth. When you throw a ball, something invisible acted on the ball forcing it to slow down, turn around, and come back down. Without that force field, you and your ball would be heading out to space right now!
Please login or register to read the rest of this content.
Please login or register to read the rest of this content.
Please login or register to read the rest of this content.
Fire is a chemical reaction (combustion) involving hot gases and plasma. The three things you need for a flame are oxygen, fuel, and a spark. When the fuel (gaseous wax) and oxygen (from the air) combine in a flame, one of the gases produced is carbon dioxide.
Most people think of carbon dioxide as dry ice, and are fascinated to watch the solid chunk sublimate from solid straight to gas, skipping the liquid state altogether. You’ve seen the curls of dry ice vapor curl down and cover the floor in a thick, wispy fog. Is carbon dioxide always more dense than air, or can we get it to float?
 Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!
Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!
SPECIAL NOTE: This instrument is NOT for looking at the sun. Do NOT look directly at the sun. But you can point the tube at a sheet of paper that has the sun’s reflected light on it.
Usually you need a specialized piece of material called a diffraction grating to make this instrument work, but instead of buying a fancy one, why not use one from around your house? Diffraction gratings are found in insect (including butterfly) wings, bird feathers, and plant leaves. While I don’t recommend using living things for this experiment, I do suggest using an old CD.
CDs are like a mirror with circular tracks that are very close together. The light is spread into a spectrum when it hits the tracks, and each color bends a little more than the last. To see the rainbow spectrum, you’ve got to adjust the CD and the position of your eye so the angles line up correctly (actually, the angles are perpendicular).
You’re looking for a spectrum (the rainbow image at left) – this is what you’ll see right on the CD itself. Depending on what you look at (neon signs, chandeliers, incandescent bulbs, fluorescent bulbs, Christmas lights…), you’ll see different colors of the rainbow. For more about how diffraction gratings work, click here.
Materials:
- old CD
- razor
- index card
- cardboard tube
This experiment is for advanced students. Did you know that eating a single peanut will power your brain for 30 minutes? The energy in a peanut also produces a large amount of energy when burned in a flame, which can be used to boil water and measure energy.
Peanuts are part of the bean family, and actually grows underground (not from trees like almonds or walnuts). In addition to your lunchtime sandwich, peanuts are also used in woman’s cosmetics, certain plastics, paint dyes, and also when making nitroglycerin.
What makes up a peanut? Inside you’ll find a lot of fats (most of them unsaturated) and antioxidants (as much as found in berries). And more than half of all the peanuts Americans eat are produced in Alabama. We’re going to learn how to release the energy inside a peanut and how to measure it.
This experiment is for advanced students.
Sparks flying off in all directions…that’s fun. In this lab, we will show how easy it is to produce those shooting sparks. In a sparkler you buy at the store, the filings used are either iron or aluminum.
The filings are placed in a mixture that, when dry, adheres to the metal rod or stick that is used in making the sparkler. The different colors are created by adding different powdered chemicals to the mixture before it dries. When they burn, we get red, blue, white, and green.
This is the experiment that your audience will remember from your chemistry magic show. Here's what happens - you call up six 'helpers' and hand each a seemingly empty test tube. Into each test tube, pour a little of the main gold-colored solution, say a few magic words, and their test tubes turn clear, black, pink, gold, yellow, and white. With a flourish, ask them to all pour their solutions back into yours and the final solution turns from inky black to clear. Voila!
I first saw a similar experiment when I was a kid, and I remembered it all the way through college, where I asked my professor how I could duplicate the experiment on my own. I was told that the chemicals used in that particular experiment were way too dangerous, and no substitute experiment was possible, especially for the color reversal at the end. I was determined to figure out an alternative. After two weeks of nothing but chemistry and experiment testing, I finally nailed it - and the best part is, you have most of these chemicals at the grocery store. (And the best part is, I can share it with you as I've eliminated the nasty chemicals so you don't have to worry about losing an eyeball or a finger.)
NOTE: This experiment requires adult help, as it uses chemicals that are toxic if randomly mixed together. Follow the instructions carefully, and do not mix random chemicals together.
Are you ready to mix up your own rainbow?
First discovered in 1886 by Hans Heinrich Landolt, the iodine clock reaction is one of the best classical chemical kinetics experiments. Here’s what to expect: Two clear solutions are mixed. At first there is no visible reaction, but after a short time, the liquid suddenly turns dark blue.
Usually, this reaction uses a solution of hydrogen peroxide with sulfuric acid, but you can substitute a weaker (and safer) acid that works just as well: acetic acid (distilled white vinegar). The second solution contains potassium iodide, sodium thiosulfate (crystals), and starch (we’re using a starch packing peanut, but you can also use plain old cornstarch). Combine one with the other to get the overall reaction, but note that there are actually two reactions happening simultaneously.
This experiment is for advanced students.
In gas form, element #59 is deadly. However, when iodine is in liquid form, it helps heal cuts and scrapes. The iodine molecule occurs in pairs, not as a single atom (many halogens do this, and it’s called a diatomic molecule). It’s hard to find iodine in nature, though it’s essential for staying healthy… too little iodine in the body takes a heavy toll on how well the brain operates.
A chunk of iodine is blackish-blue, and will sublimate (go from a solid straight to a gas). Iodine is the heaviest element needed by living things. Iodized salt is sodium chloride fortified with iodine to prevent people from not getting enough iodine in their daily diets.
Iodine is found in seaweed (kelp) and seafood as well as vegetables that are grown in dirt that has high iodine levels. People that live inland and do not eat fish often have lower iodine levels than their coastal, fish-eating neighbors. The trick is not to get too much or too little iodine in your diet, because the symptoms of deficiency and excess levels are quite similar.
Starch (like cornstarch) are used as an indicator for detecting iodine in chemistry experiments. When combined with iodine, starch forms a blue-black color in the solution. We’re going to do this and many other activities in this lab, because this experiment is actually several labs rolled into one. First, we have to make iodine, store it, and then we get to use it in several experiments. Are you ready?
Supercooling a liquid is a really neat way of keeping the liquid a liquid below the freezing temperature. Normally, when you decrease the temperature of water below 32oF, it turns into ice. But if you do it gently and slowly enough, it will stay a liquid, albeit a really cold one!
In nature, you’ll find supercooled water drops in freezing rain and also inside cumulus clouds. Pilots that fly through these clouds need to pay careful attention, as ice can instantly form on the instrument ports causing the instruments to fail. More dangerous is when it forms on the wings, changing the shape of the wing and causing the wing to stop producing lift. Most planes have de-icing capabilities, but the pilot still needs to turn it on.
We’re going to supercool water, and then disturb it to watch the crystals grow right before our eyes! While we’re only going to supercool it a couple of degrees, scientists can actually supercool water to below -43oF!
Dissolving calcium chloride is highly exothermic, meaning that it gives off a lot of heat when mixed with water (the water can reach up to 140oF, so watch your hands!). The energy released comes from the bond energy of the calcium chloride atoms, and is actually electromagnetic energy.
When you combine the calcium chloride and sodium carbonate solutions, you form the new chemicals sodium chloride (table salt) and calcium carbonate. Both of these new chemicals are solids and “fall out” of the solution, or precipitate. If you find that there is still liquid in the final solution, you didn’t have quite a saturation solution of one (or both) initial solutions.
Did you know that supercooled liquids need to heat up in order to freeze into a solid? It’s totally backwards, I know…but it’s true! Here’s the deal:
A supercooled liquid is a liquid that you slowly and carefully bring down the temperature below the normal freezing point and still have it be a liquid. We did this in our Instant Ice experiment.
Since the temperature is now below the freezing point, if you disturb the solution, it will need to heat up in order to go back up to the freezing point in order to turn into a solid.
When this happens, the solution gives off heat as it freezes. So instead of cold ice, you have hot ice. Weird, isn’t it?
Sodium acetate is a colorless salt used making rubber, dying clothing, and neutralizing sulfuric acid (the acid found in car batteries) spills. It’s also commonly available in heating packs, since the liquid-solid process is completely reversible – you can melt the solid back into a liquid and do this experiment over and over again!
The crystals melt at 136oF (58oC), so you can pop this in a saucepan of boiling water (wrap it in a towel first so you don’t melt the bag) for about 10 minutes to liquify the crystals.
This experiment is for advanced students. This is a repeat of the experiment: Can Fish Drown? but now we’re going to do this experiment again with your new chemistry glassware.
The aquarium looked normal in every way, except for the fish. They were breathing very fast and sinking head first to the bottom of the tank. They would sink a few inches, then jerk into proper movement again.
The student had to figure out what was wrong. He had set up the aquarium as an ongoing science project, and it was his responsibility to maintain the fish tank. His grade depended on it.
He went to his mom for help. She looked over the setup. “Have you tested the water?”
A quizzical look on his face, the boy said, “Everything is normal nitrates, nitrites, hardness, alkalinity, and pH. The pH was a little acidic, but not outside the proper range.”
This experiment is for advanced students.
This time we’re going to use a lot of equipment… really break out all the chemistry stuff. We’ll need all this stuff to generate oxygen with potassium permanganate (KMNO4). We will work with this toxic chemical and we will be careful…won’t we?
A ferrofluid becomes strongly magnetized when placed in a magnetic field. This liquid is made up of very tiny (10 nanometers or less) particles coated with anti-clumping surfactants and then mixed with water (or solvents). These particles don’t “settle out” but rather remain suspended in the fluid.
The particles themselves are made up of either magnetite, hematite or iron-type substance.
Ferrofluids don’t stay magnetized when you remove the magnetic field, which makes them “super-paramagnets” rather than ferromagnets. Ferrofluids also lose their magnetic properties at and above their Curie temperature points.
Ferrofluids are what scientists call “colloidal suspensions”, which means that the substance has properties of both solid metal and liquid water (or oil), and it can change phase easily between the two. (We as show you this in the video below.) Because ferrofluids can change phases when a magnetic field is applied, you’ll find ferrofluids used as seals, lubricants, and many other engineering-related uses.
Here’s a video on toner cartridges and how to make your own homemade ferrofluid. It’s a bit longer than our usual video, but we thought you’d enjoy the extra content.
This experiment is for advanced students.
Purple and white colors, making the whitewash that Tom Sawyer used, and produce an exothermic chemical reaction…..does it get any better?
Limewater is one of the compounds we work with in this experiment. Limewater was used in the old days of America. We’re talking about the 80’s…..the 1880’s.
Traveling medicine shows sold what was called “patent medicines”. These usually had no medicinal properties at all. The man in charge, the salesman of the operation, was called a “huckster”. He would have the one of the people gathered around to listen to him blow into limewater. Their exhaled breath contains carbon dioxide, and the lime water turned cloudy, just like in our experiment.
The man would hold up the glass with the cloudy limewater in it and pour in some of his fantastic remedy. As long as the “medicine” was acidic, it would turn the cloudy limewater clear. This was proof that the remedy would cure whatever ailed the person.
This experiment is for advanced students.
Zinc (Zn), is a metal and it is found as element #30 on the periodic table. We need a little zinc to keep our bodies balanced, but too much is very dangerous.
Zinc is just like the common, everyday substance that we all know as di-hydrogen monoxide (which is the chemical name for water). We need water to survive, but too much will kill us.
DHMO: In chemistry, “Di” equals the number 2; hydrogen is H; mono equals the number one; and oxide is derived from oxygen, and its symbol is O. Put these together and you have Di-hydrogen (H2), and mono oxygen (O). Put them together, what do you have? Water!
 This experiment below is for advanced students. If you’ve ever wondered why hydrogen peroxide comes in dark bottles, it’s because the liquid reacts with sunlight to decompose from H2O2 (hydrogen peroxide) into H2O (water) and O2 (oxygen). If you uncap the bottle and wait long enough, you’ll eventually get a container of water (although this takes a LOOONG time to get all of the H2O2 transformed.)
This experiment below is for advanced students. If you’ve ever wondered why hydrogen peroxide comes in dark bottles, it’s because the liquid reacts with sunlight to decompose from H2O2 (hydrogen peroxide) into H2O (water) and O2 (oxygen). If you uncap the bottle and wait long enough, you’ll eventually get a container of water (although this takes a LOOONG time to get all of the H2O2 transformed.)
Here’s a way to speed up the process and decompose it right before your eyes. For younger kids, you can modify this advanced-level experiment so it doesn’t involve flames. Here’s what you do:
 If you’ve ever owned a fish tank, you know that you need a filter with a pump. Other than cleaning out the fish poop, why else do you need a filter? (Hint: think about a glass of water next to your bed. Does it taste different the next day?)
If you’ve ever owned a fish tank, you know that you need a filter with a pump. Other than cleaning out the fish poop, why else do you need a filter? (Hint: think about a glass of water next to your bed. Does it taste different the next day?)
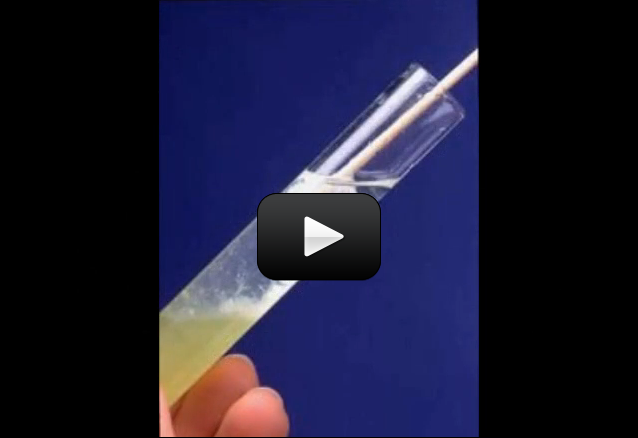
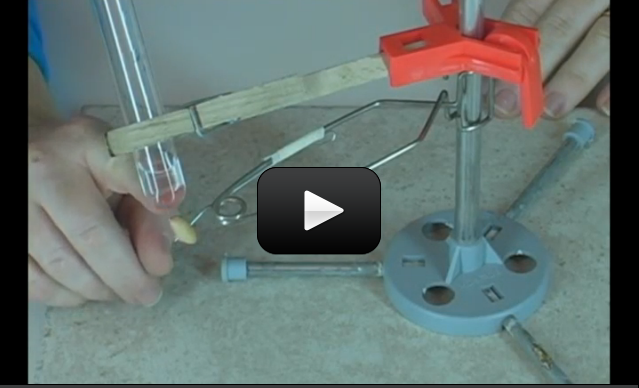
There are tiny air bubbles trapped inside the water, and you can see this when you boil a pot of water on the stove. The experimental setup shown in the video illustrates how a completely sealed tube of water can be heated… and then bubbles come out one end BEFORE the water reaches a boiling point. The tiny bubbles smoosh together to form a larger bubble, showing you that air is dissolved in the water.
Materials:
- test tube clamp
- test tube
- lighter (with adult help)
- alcohol burner or votive candle
- right-angle glass tube inserted into a single-hole stopper
- regular tap water