If you’ve been following the energy curriculum up to this point and are looking forward to more great energy stuff, stay tuned. Electromagnetic energy is coming soon. However, before we get there, we need to take a bit of a side road. We need to wander into the world of quantum physics for a bit and take a look at the teeny and the mysterious world of atoms.We’re going to study atoms, their parts, as well as how they work together. Are you ready?
You can get started by watching the video, and afterward either read more about it or start your experiments!
Scientific Concepts for Atoms:
- All matter is made of atoms.
- An atom is the smallest part of stable matter.
- Atoms rarely hang out alone. They join together in groups from two to millions of atoms.
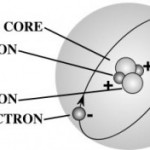
- Atoms are made of three basic particles. Neutrons, protons, and electrons.
- Neutrons and protons are together in the middle of the atom and make up the nucleus of the atom. Electrons move around the nucleus. They don’t “orbit” the nucleus. Next lesson we will talk more about how they move. It’s one of the wacky things about electrons.
- Atoms differ from one another by how many protons, neutrons, and electrons they have in them.
- Elements are specific kinds of atoms. Every atom is a type of element.
- There are over 112 elements. Ninety of which are found naturally. Twelve different elements are the major ingredients of over 90% of all matter. Five different elements are the major ingredients of all living things.
- Carbon, Hydrogen, Oxygen, Nitrogen, and Calcium are the five main elements that make up all living matter.
- Most atoms come from stars and have been around since the beginning of time.
- Atoms get used, and reused again and again as things change over time.
Scientific Concepts for Electrons:
- Electrons don’t orbit nuclei. They pop in and pop out of existence.
- Electrons do tend to stay at a certain distance from a nucleus. This area that the electron tends to stay in is called a shell.
- The electrons move so fast around the shell that the shell forms a balloon like ball around the nucleus.
- An atom can have as many as seven shells.
- The number of electrons an atom has determines how many shells it has.
- A shell can only hold so many electrons. The number of electrons a shell can hold can be determined by the formula 2n2 where n is the number of the shell.
- Atoms are “satisfied” if they have a full outer shell or if they have a multiple of eight electrons in their outer shell.
- If an atom is not “satisfied” it will gladly share electrons with other atoms forming molecules.
Scientific Concepts for Density:
- Density is a measurement of mass and volume.
- The denser something is the tighter its atoms are packed together.
- Mathematically, density is mass/volume.