The summer chemistry section is full of great experiments that are not only fun but also designed to hone your observational skills. Chemistry experiments really speak for themselves, much better than I can ever put into words. And I’m going to provide you with a lot of these experiments to help you develop your observing techniques. Are you ready?
|
Simple Spectrometer and the more advanced Calibrated Spectrometer
|
|||||
 |
As promised, here’s the Liquid Nitrogen Ice Cream Social video that was only available to a handful of participants at our live summer camp last week! This is probably one of the last times Dr. Tom Frey will be doing this presentation, so we didn’t want to miss the opportunity to record it and share it with you!
Dr. Tom Frey just retired as a chemistry professor at Cal Poly State University, where he taught courses (including how to make your own lab glassware) for 42 years. He’s not only a mentor of mine, but a close personal friend and I am happy to share his talent and passion for science with you through this special, one of a kind video.
I really hope you enjoy it!
Please login or register to read the rest of this content.
A ferrofluid becomes strongly magnetized when placed in a magnetic field. This liquid is made up of very tiny (10 nanometers or less) particles coated with anti-clumping surfactants and then mixed with water (or solvents). These particles don’t “settle out” but rather remain suspended in the fluid.
The particles themselves are made up of either magnetite, hematite or iron-type substance.
Ferrofluids don’t stay magnetized when you remove the magnetic field, which makes them “super-paramagnets” rather than ferromagnets. Ferrofluids also lose their magnetic properties at and above their Curie temperature points.
Ferrofluids are what scientists call “colloidal suspensions”, which means that the substance has properties of both solid metal and liquid water (or oil), and it can change phase easily between the two. (We as show you this in the video below.) Because ferrofluids can change phases when a magnetic field is applied, you’ll find ferrofluids used as seals, lubricants, and many other engineering-related uses.
Here’s a video on toner cartridges and how to make your own homemade ferrofluid. It’s a bit longer than our usual video, but we thought you’d enjoy the extra content.
Please login or register to read the rest of this content.
You’d be surprised at where vitamin C pops up in your kitchen – I know I was! I found it in my broccoli, butternut squash, sweet potatoes, swiss chard, spinach, carrots, kale, peas, leeks, tomatoes, guava, watermelon, grapefruit, pecans, raspberries, bell peppers, onion, papaya, pineapple, and pistachios!
Vitamin C is essential for humans and some animals (mostly primates), protecting the body from oxidative stress (involved in many diseases) and helping heal wounds. When humans don’t get enough vitamin C, they get sick with scurvy (bleeding from all mucus membranes, brown spots on the skin…), which is actually how scientists discovered vitamin C in the first place.
Plants and most animals actually make their own vitamin C by converting glucose (sugar).
Latest research (2008) discovered that the red blood cells in humans and primates more efficiently use vitamin C already in the body by recycling DHA. The neat part about t his discovery is that only humans and some primates have this ability – plants and most other animals do not.
Let’s find out how much of this vitamin is already in your home – are you ready?
There are two videos to watch – the first shows you how to make your own homemade indicator, and the second shows you how to use it.
Please login or register to read the rest of this content.
Supercooling a liquid is a really neat way of keeping the liquid a liquid below the freezing temperature. Normally, when you decrease the temperature of water below 32oF, it turns into ice. But if you do it gently and slowly enough, it will stay a liquid, albeit a really cold one!
In nature, you’ll find supercooled water drops in freezing rain and also inside cumulus clouds. Pilots that fly through these clouds need to pay careful attention, as ice can instantly form on the instrument ports causing the instruments to fail. More dangerous is when it forms on the wings, changing the shape of the wing and causing the wing to stop producing lift. Most planes have de-icing capabilities, but the pilot still needs to turn it on.
We’re going to supercool water, and then disturb it to watch the crystals grow right before our eyes! While we’re only going to supercool it a couple of degrees, scientists can actually supercool water to below -43oF!
Please login or register to read the rest of this content.
Did you know that supercooled liquids need to heat up in order to freeze into a solid? It’s totally backwards, I know…but it’s true! Here’s the deal:
A supercooled liquid is a liquid that you slowly and carefully bring down the temperature below the normal freezing point and still have it be a liquid. We did this in our Instant Ice experiment.
Since the temperature is now below the freezing point, if you disturb the solution, it will need to heat up in order to go back up to the freezing point in order to turn into a solid.
When this happens, the solution gives off heat as it freezes. So instead of cold ice, you have hot ice. Weird, isn’t it?
Sodium acetate is a colorless salt used making rubber, dying clothing, and neutralizing sulfuric acid (the acid found in car batteries) spills. It’s also commonly available in heating packs, since the liquid-solid process is completely reversible – you can melt the solid back into a liquid and do this experiment over and over again!
The crystals melt at 136oF (58oC), so you can pop this in a saucepan of boiling water (wrap it in a towel first so you don’t melt the bag) for about 10 minutes to liquify the crystals.
Please login or register to read the rest of this content.
Did you know that most people can’t crack an egg with only one hand without whacking it on something? The shell of an egg is quite strong! Try this over a sink and see if you can figure out the secret to cracking an egg in the palm of your hand…(Hint: the answer is below the video – check it out after you’ve tried it first!)
How can you tell if an egg is cooked or raw? Simply spin it on the counter and you’ll get a quick physics lesson in inertia…although you might not know it. A raw egg is all sloshy inside, and will spin slow and wobbly. A cooked egg is all one solid chunk, so it spins quickly. Remember the Chicken and the Clam experiment?
Please login or register to read the rest of this content.
If you soak chicken bones in acetic acid (distilled vinegar), you’ll get rubbery bones that are soft and pliable as the vinegar reacts with the calcium in the bones. This happens with older folks when they lose more calcium than they can replace in their bones, and the bones become brittle and easier to break. Scientists have discovered calcium is replaced more quickly in bodies that exercise and eating calcium rich foods, like green vegetables.
This is actually two experiments in one – here’s what you need to do:
Please login or register to read the rest of this content.
 Have you ever tried washing dishes without soap? It doesn’t work well, especially if there’s a lot of grease, fat, or oil on the dish!
Have you ever tried washing dishes without soap? It doesn’t work well, especially if there’s a lot of grease, fat, or oil on the dish!
The oils and fats are slippery and repel water, which makes them a great choice for lubration of bearing and wheels, but lousy for cleaning up after dinner.
So what’s inside soap that makes it clean off the dish? The soap molecule looks a lot like a snake, with a head and a tail. The long tail loves oil (hydrophobic) and the head loves water (hydrophilic). The hydrophilic end dissolves in water and the hydrophobic end wraps itself around fat and oil in the dirty water, cleaning it off your dishes.
Let’s do an experiment that will really make you appreciate soap and fat:
Please login or register to read the rest of this content.
A combustion reaction gives off energy, usually in the form of heat and light. The reaction itself includes oxygen combining with another compound to form water, carbon dioxide, and other products.
A campfire is an example of wood and oxygen combining to create ash, smoke, and other gases. Here’s the reaction for the burning of methane (CH4) which gives carbon dioxide (CO2) and water (H2O):
Please login or register to read the rest of this content.
When you think of slime, do you imagine slugs, snails, and puppy kisses? Or does the science fiction film The Blob come to mind? Any way you picture it, slime is definitely slippery, slithery, and just plain icky — and a perfect forum for learning real science.
But which ingredients work in making a truly slimy concoction, and why do they work? Let’s take a closer look!
Imagine a plate of spaghetti. The noodles slide around and don’t clump together, just like the long chains of molecules (called polymers) that make up slime. They slide around without getting tangled up. The pasta by itself (fresh from the boiling water) doesn’t hold together until you put the sauce on. Slime works the same way. Long, spaghetti-like chains of molecules don’t clump together until you add the sauce … until you add something to cross-link the molecule strands together.
Please login or register to read the rest of this content.
This is a Bonus Lab, which means that the experiments in this section require adult help (we’re working with fire in several of them), and/or the materials are more expensive and hard to find.
Use the experiments in this section for kids wanting to go even further and deeper into the subject. Since these are more involved, be sure to browse through the videos for these experiments first before purchasing materials for these additional labs.
You do not need to do ALL the experiments – just pick the ones you want to do! Look over the experiments and note which items are needed, and off you go!
Click here for a printer-friendly version of this page.
Materials for Kitchen Chemistry
- Milk (whole or lowfat)
- Food dye
- Dish soap
- Water and Ice
- Bowl
- Drinking glass
- Egg (two hardboiled)
- Vinegar
- Salt
- Hydrogen peroxide
- Yeast (the kind you use for bread)
- Empty water or soda bottle (1 liter size)
- Cornstarch (a couple tablespoons)
- Iodine (from the pharmacy)
- Fresh citrus for testing
- Popsicle sticks or disposable plastic spoon
- Acetone (fingernail polish remover)
- Styrofoam cup
Materials for Bubblology
- Straws
- String
- Paper clips (small)
- 2L soda bottles
- Plastic berry basket
- Wire coat hangers (bendable)
- Thick rubber bands
- Stiff card stock (or paper)
- Plate or cookie sheet
- Balloon
- 6 feet of loosely woven inch-wide fabric trim (lace)
- Scissors
- Water (distilled if you have it)
- 1-2 cups clear Ivory dish soap OR liquid Joy Ultra OR green Dawn
- Buckets to hold your soap solution
- Glycerin (check your pharmacy)
Materials for Acids and Bases
- pH paper OR a head of red cabbage and paper towels/coffee filter
- Juice or fruit (anything you have will work)
Materials for Instant Crystal Sculptures
- Reusable hand warmer (the kind with a metal disc inside you flex to activate the sodium acetate)
- Disposable plate
- Scissors
Materials for Liquid Magnets
- Vegetable cooking oil (1/4 cup)
- Old toner or liquid toner
- Magnet
- Small soda bottle with cap
Materials for Volcanoes
- 9 cups flour
- 3 cups dirt
- 4 cups salt
- 1 cup sand
- Water
- Disposable roasting pan
- 4 cups baking soda
- 4 cups distilled white vinegar
- 2 empty water bottles
- 1 cup liquid dish soap
- 1 cup aluminum sulfate (check gardening section)
- 18” length of clear, flexible tubing (any diameter between ¼” – ½”)
- Red food dye (optional)
Materials for Water Purification Experiment
- coffee with grinds mixed back in stored in an old plastic water bottle
- popsicle sticks for mixing
- activated carbon granules (from a fish tank supply store)
- clean water
- funnel
- two cotton balls
- three small disposable cups (clear is best so you can see what you’re doing)
- medicine dropper or syringe (no needle)
- aluminum sulfate (AKA alum from the spice section)
- calcium hydroxide (AKA lime) from the gardening section. Note: keep this chemical packed away, as the dust is toxic and should not be inhaled.
Materials for Slime Science
- yellow highlighter pen
- guar gum (check health food stores)
- sodium tetraborate (AKA: borax)
- liquid starch (check the laundry aisle for Vano or Sta-Flo)
- cornstarch (about 2 cups)
- white glue
- clear glue
- disposable cups
- popsicle sticks
- measuring spoons
- water
- sugar (about 1 cup)
- goggles
- PVA (polyvinyl alcohol) (optional)
- food dye (optional)
Materials for Bouncy Balls
- Sodium Silicate (from Unit 3)
- Ethyl Alcohol (check your pharmacy)
- 2 disposable cups (don’t use your kitchen glassware, as you’ll never get it clean again)
- 2 Popsicle sticks (again, use something disposable to stir with)
- Gloves for your hands
- Goggles for your face
Materials for Burning Money
- Shallow baking dish
- Tongs
- Rubbing Isopropyl Alcohol (50-91%)
- Dollar bill
- Fire extinguisher with adult help
Materials for Football Ice Cream:
- 1 quart whole milk (do not substitute, unless your child has a milk allergy, use soy or almond milk)
- 1 pint heavy cream (do not substitute, unless your child has a milk allergy, then skip)
- 1 cup sugar (or other sweetener)
- 1 tsp vanilla (use non-alcohol kind)
- Rock salt (use table salt if you can’t find it)
- Lots of ice
- Freezer-grade zipper-style bags (you’ll need quart and gallon sizes)
Materials for Colored Campfires & Spectrometer
- Old ceramic or metal pot with lid
- Heat-proof surface
- BBQ lighter with adult help
- Methanol
- Popsicle sticks
Select the chemical additive you want:
- Boric acid
- Sodium tetraborate (borax)
- Epsom salts (magnesium sulfate)
- Regular table salt (sodium chloride)
- Salt substitute (potassium chloride)
- Ice Melt or Dri-Ez (pure calcium chloride)
To build the spectrometer:
- Cardboard box (ours is 10″ x 5″ x 5″, but anything close to this will work fine)
- Diffraction grating (you can order a sheet here)
- Two razor blades (with adult help)
- Masking tape
- Ruler
- Photocopy of a cm (centimeter) ruler (or sketch a line with 1 through 10 cm markings on it, about 4cm wide)
Materials for Iodine Rainbow
- Iodine (clear, non-ammonia from the pharmacy)
- Hydrogen peroxide (3% solution)
- Vinegar (distilled white is best)
- Cornstarch (tiny pinch) or one starch packing peanut
- Water
- Sodium Thiosulfate
- Sodium Carbonate (AKA: “washing soda“)
- Phenolphthalein (keep this out of reach of kids)
- 6 disposable cups
- 6 Popsicle sticks
- Gloves for your hands
- Goggles for your face
Instead of using glue as a polymer (as in the slime recipes above), we're going to use PVA (polyvinyl alcohol). Most liquids are unconnected molecules bouncing around. Monomers (single molecules) flow very easily and don't clump together. When you link up monomers into longer segments, you form polymers (long chains of molecules).
Polymers don't flow very easily at all - they tend to get tangled up until you add the cross-linking agent, which buddies up the different segments of the molecule chains together into a climbing-rope design.
Squishy Slime Mix 1 cup sugar, 12 cups water, and 3 cups cornstarch in a saucepan. Stir constantly over medium heat until thickened, about 5 minutes. Place a glop in each of several bowls along with drops of food coloring in each. Place a dollop of each color into a plastic sandwich bad and zip it shut. You can squish and squeeze without getting your hands slimy!
Please login or register to read the rest of this content.
Ever wonder why ketchup doesn't flow easily out of the bottle? Now you know it's because the ketchup acts just like the cornstarch-water experiment here. More examples of non-Newtonian fluids are ketchup, blood, paint, and shampoo.
We're going to whip up a batch of non-Newtonian fluid that's going to act like both a solid and a liquid. Here's what you do:
The glue is a polymer, which is a long chain of molecules all hooked together like tangled noodles. When you mix the two solutions together, the water molecules start linking up the noodles together all along the length of each noodle to get more like a fishnet. Scientists call this a polymetric compound of sodium tetraborate and lactated glue. We call it bouncy putty.
Here’s what you do:
Please login or register to read the rest of this content.
Is it hot where you live in the summer? What if I gave you a recipe for making ice cream that doesn’t require an expensive ice cream maker, hours of churning, and can be made to any flavor you can dream up? (Even dairy-free if needed?)
If you’ve got a backyard full of busy kids that seem to constantly be in motion, then this is the project for you. The best part is, you don’t have to do any of the churning work… the kids will handle it all for you!
This experiment is simple to set up (it only requires a trip to the grocery store), quick to implement, and all you need to do guard the back door armed with a hose to douse the kids before they tramp back into the house afterward.
One of the secrets to making great ice cream quickly is Please login or register to read the rest of this content.
Always have a FIRE EXTINGUISHER and ADULT HELP handy when performing fire experiments. NO EXCEPTIONS.
This video will show you how to transform the color of your flames. For a campfire, simply sprinkle the solids into your flames (make sure they are ground into a fine powder first) and you’ll see a color change. DO NOT do this experiment inside your house – the fumes given off by the chemicals are not something you want in your home!
One of the tricks to fire safety is to limit your fuel. The three elements you need for a flame are: oxygen, spark, and fuel. To extinguish your flames, you’ll have to either wait for the fuel to run out or smother the flames to cut off the oxygen. When you limit your fuel, you add an extra level of safety to your activities and a higher rate of success to your eyebrows.
Here’s what we’re going to do: first, make your spectrometer: you can make the simple spectrometer or the more-advanced calibrated spectrometer. Next, get your chemicals together and build your campfire. Finally, use your spectrometer to view your flames.
Please login or register to read the rest of this content.
Guar gum comes from the guar plant (also called the guaran plan), and people have found a lot of different and interesting uses for it. It’s one of the primary substitutes for fat in low-fat and fat-free foods. Cooks like to use guar gum in foods as it has 8 times the thickening power of cornstarch, so much less is needed for the recipe. Ice cream makers use it to keep ice crystals from forming inside the carton. Doctors use it as a laxative for their patients.
When we teach kids how to make slime using guar gum, they call it “fake fat” slime, mostly because it’s used in fat-free baking. You can find guar gum in health food stores or order it online. We’re going to whip up a batch of slime using this “fake fat”. Ready?
Here’s what you do:
Please login or register to read the rest of this content.
 If you’ve ever wanted to make your own version of a volcano that burps and spit all over the place, then this is the experiment for you. We used to teach kids how to make genuine Fire & Flame volcanoes, but parents weren’t too happy about the shower of sparks that hit the ceiling and fireballs that shot out of the thing… so we’ve toned it down a bit to focus more on the lava flow.
If you’ve ever wanted to make your own version of a volcano that burps and spit all over the place, then this is the experiment for you. We used to teach kids how to make genuine Fire & Flame volcanoes, but parents weren’t too happy about the shower of sparks that hit the ceiling and fireballs that shot out of the thing… so we’ve toned it down a bit to focus more on the lava flow.
Please login or register to read the rest of this content.
Ever wonder how the water draining down your sink gets clean again? Think about it: The water you use to clean your dishes is the same water that runs through the toilet. There is only one water pipe to the house, and that source provides water for the dishwasher, tub, sink, washing machine, toilet, fish tank, and water filter on the front of your fridge. And there’s only one drain from your house, too! How can you be sure what’s in the water you’re using?
This experiment will help you turn not only your coffee back into clear water, but the swamp muck from the back yard as well. Let’s get started.
Please login or register to read the rest of this content.
Ever play with a prism? When sunlight strikes the prism, it gets split into a rainbow of colors. Prisms un-mix the light into its different wavelengths (which you see as different colors). Diffraction gratings are tiny prisms stacked together.
When light passes through a diffraction grating, it splits (diffracts) the light into several beams traveling at different directions. If you’ve ever seen the ‘iridescence’ of a soap bubble, an insect shell, or on a pearl, you’ve seen nature’s diffraction gratings.
Scientist use these things to split incoming light so they can figure out what fuels a distant star is burning. When hydrogen burns, it gives off light, but not in all the colors of the rainbow, only very specific colors in red and blue. It’s like hydrogen’s own personal fingerprint, or light signature.
While this spectrometer isn't powerful enough to split starlight, it's perfect for using with the lights in your house, and even with an outdoor campfire. Next time you're out on the town after dark, bring this with you to peek different types of lights - you'll be amazed how different they really are. You can use this spectrometer with your Colored Campfire Experiment also.
SPECIAL NOTE: This instrument is NOT for looking at the sun. Do NOT look directly at the sun. But you can point the tube at a sheet of paper that has the the sun’s reflected light on it.
Here's what you do:
When I teach camp, the last experiment on Chemistry day is to ‘walk on water’. I present the kids with a milky-white tub full of water and a secret ingredient. I stick my hand in the tub and pull it out (slowly) so they can see it’s clearly a liquid (as it dribbles off my fingers), and the kids always gasp in surprise when I then smack it with my hand, because now it looks rock-solid.
Their challenge? To step up and across without sinking. Of course, sinking can be fun, too!
NOTE: You’ll see a tub full of water at the end of the line – this helps wash off their feet after they’re done stepping across.
Here’s what you need to do:
Please login or register to read the rest of this content.

If you’re fascinated by the simple complexity of the standard soap bubble, then this is the lab for you. You can easily transform these ideas into a block-party Bubble Festival, or just have extra fun in the nightly bathtub. Either way, your kids will not only learn about the science of water, molecules, and surface tension, they’ll also leave this lab cleaner than they started (which is highly unusually for science experiments!)
Soap also makes water stretchy. If you’ve ever tried making bubbles with your mouth just using spit, you know that you can’t get the larger, fist-sized spit bubbles to form completely and detach to float away in the air. Spit is 94% water, and water by itself has too much surface tension, too many forces holding the molecules together. When you add soap to it, they relax a bit and stretch out. Soap makes water stretch and form into a bubble.
Please login or register to read the rest of this content.
 Click here for a printer-friendly version of this page.
Click here for a printer-friendly version of this page.
Objective You’re going to do several chemistry experiments that expose your kids to the different type of chemical reactions. I want you to focus on honing your observational skills when you do the experiments. Did the temperature, color or volume change? Even the smallest differences can indicate something big is going on. The first thing to do is watch the video on the Chemical Demonstrations website page, and then dive into the experiments.
Main Ideas Chemistry is chocked full of demonstrations and experiments for two big reasons. First, they’re fun. But more importantly, the reason we do experiments in chemistry is to hone your observational skills. Chemistry experiments really speak for themselves, much better than I can ever put into words or show you on a video. And I’m going to hit you with a lot of these chemistry demonstrations to help you develop your observing techniques.
While the kids are playing with the experiments see if you can get them to notice these important ideas. When they can explain these concepts back to you (in their own words or with demonstrations), you’ll know that they’ve mastered the lesson.
About the Experiments A lot of folks get nervous around chemistry. You can’t always ’see’ what’s going on (are there toxic gases generated from that reaction?), and many people have a certain level of fear around chemicals in general.
I don’t want you dipping your hands in molten lead or lying on a bed of nails while someone with a sledgehammer breaks a cinder block on your stomach. Demonstrations of this kind that result in injury are the ones forever burned in the memory of the audience, who are now fearful and have made the generalization that chemicals are dangerous and their effects are bad. In fact, every chemical is potentially harmful if not handled properly. That is why I’ve prepared a special set of chemistry experiments that include step-by-step demonstrations on how to properly handle the chemicals, use them in the experiment, and dispose of them when you’re finished.
Chemistry is predictable, just as dropping a ball from a height always hits the floor. Every time you add 1 teaspoon of baking soda to 1 cup of vinegar, you get the same reaction. It doesn’t simply stop working one time and explode the next. I’m going to walk you through every step of the way, and leave you to observe the reactions and write down what you notice. At first, it’s going to seem like a lot of disjointed ideas floating around, but after awhile, you’ll start to see patterns in the way chemicals interact with each other. Keep working at Chemistry and eventually it will click into place. And if there’s an experiment you don’t want to do, just skip it (or just watch the video). Some of this may be a review for you, especially if you’ve completed Units 3 (Matter) and 8 (Chemistry 1).
The How and Why Explanation There are several different types of chemical reactions: combustion, decomposition, synthesis, and displacement. All chemical reactions somehow fit into one of these, and here’s how you can tell them apart…
Combustion: A combustion reaction gives off energy, usually in the form of heat and light. The reaction itself includes oxygen combining with another compound to form water, carbon dioxide, and other products. A campfire is an example of wood and oxygen combining to create ash, smoke, and other gases.
Synthesis: This reaction happens when simple compounds come together to form a more complicated compound. The iron (Fe) in a nail combines with oxygen (O2) to form rust, also called iron oxide (Fe2O3).
Oxidation-Reduction (Redox Reaction): When the oxidation numbers of atoms change during the reaction, it’s called a redox reaction. Oxidation happens when a compound loses electrons (increases oxidation state) and reduction occurs when a compound gains electrons (decrease in oxidation state). Electroplating is an example of a redox reaction.
Decomposition: On the other side, a decomposition reaction breaks a complicated molecule into simpler ones. When you leave a bottle of hydrogen peroxide on the counter, it decomposes into water (H2O) and oxygen (O2).
Displacement: There are several different types of displacement reactions, including single, double, and acid-base more on this later). Antacids like calcium hydroxide (CaOH) combine with stomach acid (HCl) to form calcium chloride salt (CaCl2) and water (H2O).
Questions to Ask When you’ve worked through most of the experiments ask your kids these questions and see how they do:
- What’s true about phenolphthalein? (a) it goes from clear to pink when mixed with bases (b) it’s impossible to spell (c) it is colorless in acidic solutions (d) soluble in water
- How does increasing the hydrogen peroxide affect the rate of the iodine clock reaction?
- Which chemical turns coldest when added to water? (a) calcium chloride (b) aluminum sulfate (c) ammonium nitrate (d) citric acid
- A polymer is: (a) a long piece of spaghetti (b) an element on the periodic table (c) a long molecular chain (d) a plastic bag
- What does a cross-linking agent do?
- Which of the following are cross-linking agents? (a) calcium (b) borax (c) white glue (d) starch (e) bubble gum
- Which substance is both a solid and a liquid? (a) bubble gum (b) slime (c) cornstarch and water (d) last night’s dinner
Answers:
- What’s true about phenolphthalein? (a) it goes from clear to pink when mixed with bases (b) it’s impossible to spell (c) it is colorless in acidic solutions (d) soluble in water
- How does increasing the hydrogen peroxide affect the rate of the iodine clock reaction? By accelerating the first reaction, you can shorten the time it takes the solution to change color. There are a few ways to do this: You can decrease the pH (increasing H+ concentration), or increase the iodide or hydrogen peroxide. (To lengthen the time delay, add more sodium thiosulfate.)
- Which chemical turns coldest when added to water? (a) calcium chloride (b) aluminum sulfate (c) ammonium nitrate (d) citric acid
- A polymer is: (a) a long piece of spaghetti (b) an element on the periodic table (c) a long molecular chain (d) a plastic bag
- What does a cross-linking agent do? Coagulates the polymers. (Turns the long polymer chains into something that looks more like a fishnet.)
- Which of the following are cross-linking agents? (a) calcium (b) borax (c) white glue (d) starch (e) bubble gum
- Which substance is both a solid and a liquid? (a) bubble gum (b) slime (c) cornstarch and water (d) last night’s dinner
 Click here for a printer-friendly version of this page.
Click here for a printer-friendly version of this page.
Objective When you think of slime, do you imagine slugs, snails, and puppy kisses? Or does the science fiction film The Blob come to mind? Any way you picture it, slime is definitely slippery, slithery, and just plain icky — and a perfect forum for learning real science.
Imagine a plate of spaghetti. The noodles slide around and don’t clump together, just like the long chains of molecules (called polymers) that make up slime. They slide around without getting tangled up. The pasta by itself (fresh from the boiling water) doesn’t hold together until you put the sauce on. Slime works the same way. Long, spaghetti-like chains of molecules don’t clump together until you add the sauce … until you add something to cross-link the molecule strands together.
About the Experiment To make our different slimes, we’ll be using borax as the cross-linking agent. There a lots of different polymers you can try, including starch, glue, and polyvinyl alcohol. The polymer (usually glue) mixture is the “spaghetti” (the long chain of molecules), and the “sauce” is the borax mixture (the cross-linking agent). You need both in order to create slime. Keep your slime in the fridge for a week, or a month in the freezer (although it might change colors). Nuke it in the microwave for a few seconds to thaw.
The How and Why Explanation The cross-linking agent in the slime mixtures can be either liquid starch or borax (sodium tetraborate). When you mix the glue and water together, you’ve got a cup full of long molecule chains, like a pile of ropes. When you cross-link the polymers, it’s like building a net with the rope, and it happens very quickly to give you that rubbery, stretchy substance kids are so fond of.
Questions to Ask When you’ve worked through most of the experiments ask your kids these questions and see how they do:
- A polymer is: (a) a long piece of spaghetti (b) an element on the periodic table (c) a long molecular chain (d) a plastic bag
- What does a cross-linking agent do?
- Which of the following are cross-linking agents? (a) calcium (b) borax (c) white glue (d) starch (e) guar gum
- What does PVA stand for? What kind of water does it mix with?
- Which substance is both a solid and a liquid? (a) guar gum (b) bouncy putty (c) starch slime (d) corny slime
 Click here for a printer-friendly version of this page.
Click here for a printer-friendly version of this page.
Objective This experiment will teach you about the different types of filtration as you turn your coffee back into clear water as well as the swamp muck from the back yard. You can test out other types of “swamp muck” by mixing together other liquids (water, orange juice, etc.) and solids (citrus pulp, dirt, etc.). Stay away from carrot juice, grape juice, and beets — they won’t work with this type of filter.
About the Experiment Ever wonder how the water draining down your sink gets clean again? Think about it: The water you use to clean your dishes is the same water that runs through the toilet. There is only one water pipe to the house, and that source provides water for the dishwasher, tub, sink, washing machine, toilet, fish tank, and water filter on the front of your fridge. And there’s only one drain from your house, too! How can you be sure what’s in the water you’re using?
In our live Science Camp Workshops during the summer, we let the kids bring in their own samples which usually includes a blenderized version of leftovers from dinner, plant trimmings, coffee grounds, and dirt. It’s quite a smelly class once everyone cracks open their samples!
The How and Why Explanation There are several steps to understand as we go along:
- Aeration: Aerate water to release the trapped gas. You do this in the experiment by pouring the water from one cup to another.
- Coagulation: Alum collects small dirt particles, forming larger, sticky particles called floc.
- Sedimentation: The larger floc particles settle to the bottom of the cup.
- Filtration: The smaller floc particles are trapped in the layer of sand and cotton.
- Disinfection: A small amount of disinfectant is added to kill the remaining bacteria. This is for informational purposes only — we won’t be doing it in this experiment.
Questions to Ask When you’ve worked through most of the experiments ask your kids these questions and see how they do:
- What is the alum used for in the water filtration experiment? (a) to adjust the pH (b) to form floc (c) to float to the top (d) to purify the sample
- What does the activated carbon do? (a) turns the cotton balls black (b) sinks the floc (c) adjusts the pH (d) adds to the filtering power of the cotton (e) none of the above (f) all of the above
- Draw a sketch of your filter, labeling the different layers.

Click here for a printer-friendly version of this page.
Objective If you’re fascinated by the simple complexity of the standard soap bubble, then this is the lab for you. You can easily transform these ideas into a block-party Bubble Festival, or just have extra fun in the nightly bathtub. Either way, your kids will not only learn about the science of water, molecules, and surface tension, they’ll also leave this lab cleaner than they started (which is highly unusually for science experiments!)
About the Experiments The absolute best time to make gigantic bubbles is on an overcast day, right after it rains. Bubbles have a thin cell wall that evaporates quickly in direct sun, especially on a low-humidity day. If you live in a dry area with low-humidity, be sure to use glycerin. The glycerin will add moisture and deter the rapid thinning of the bubble’s cell wall (which cause bubbles to tear and pop).
The How and Why Explanation If you pour a few droplets of water onto a sweater or fabric, you’ll notice that the water will just sit there on the surface in a ball (or oval, if the drop is large enough). If you touch the ball of water with a soapy finger, the ball disappears into the fibers of the fabric! What happened?
Soap makes water “wetter” by breaking down the water’s surface tension by about two-thirds. Surface tension is the force that keeps the water droplet in a sphere shape. It’s the reason you can fill a cup of water past the brim without it spilling over. Without soap, water can’t get into the fibers of your clothes to get them clean. That’s why you need soap in the washing machine.
Soap also makes water stretchy. If you’ve ever tried making bubbles with your mouth just using spit, you know that you can’t get the larger, fist-sized spit bubbles to form completely and detach to float away in the air. Spit is 94% water, and water by itself has too much surface tension, too many forces holding the molecules together. When you add soap to it, they relax a bit and stretch out. Soap makes water stretch and form into a bubble.
The soap molecule looks a lot like a snake; it’s a long chain that has two very different ends. The head of the snake loves water, and the tail loves dirt. When the soap molecule finds a dirt particle, it wraps its tail around the dirt and holds it.
The different colors of a soap bubble come from how the white light bounces off the bubble into your eye. Some of the light bounces off the top surface of the bubble and bends only a little bit, while the rest passes through the thin film and bounces off the inner surface of the bubble and refracts more.
If you made the Hover Bubbles, you’ll notice that the bubbles slowly get larger the longer they live in the tank. Remember that the bubble is surrounded by CO2 gas as it sinks. The bubble grows because carbon dioxide seeps through the bubble film faster than the air seeps out, as CO2 is more soluble in water than air (meaning that CO2 mixes more easily with water than air does).
Questions to Ask
- Which soap solution makes the biggest bubbles?
- Does water temperature matter?
- How would you make a square bubble?
- Why can you poke a straw through a bubble after it’s been dipped in bubble solution?
 Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!
Spectrometers are used in chemistry and astronomy to measure light. In astronomy, we can find out about distant stars without ever traveling to them, because we can split the incoming light from the stars into their colors (or energies) and “read” what they are made up of (what gases they are burning) and thus determine their what they are made of. In this experiment, you’ll make a simple cardboard spectrometer that will be able to detect all kinds of interesting things!
SPECIAL NOTE: This instrument is NOT for looking at the sun. Do NOT look directly at the sun. But you can point the tube at a sheet of paper that has the sun’s reflected light on it.
Usually you need a specialized piece of material called a diffraction grating to make this instrument work, but instead of buying a fancy one, why not use one from around your house? Diffraction gratings are found in insect (including butterfly) wings, bird feathers, and plant leaves. While I don’t recommend using living things for this experiment, I do suggest using an old CD.
CDs are like a mirror with circular tracks that are very close together. The light is spread into a spectrum when it hits the tracks, and each color bends a little more than the last. To see the rainbow spectrum, you’ve got to adjust the CD and the position of your eye so the angles line up correctly (actually, the angles are perpendicular).
You’re looking for a spectrum (the rainbow image at left) – this is what you’ll see right on the CD itself. Depending on what you look at (neon signs, chandeliers, incandescent bulbs, fluorescent bulbs, Christmas lights…), you’ll see different colors of the rainbow. For more about how diffraction gratings work, click here.
Materials:
- old CD
- razor
- index card
- cardboard tube
This is one of those 'chemistry magic show' type of experiments to wow your friends and family. Here's the scoop: you take a cup of clear liquid, add it to another cup of clear liquid, stir for ten seconds, and you'll see a color change, a state change from liquid to solid, and you can pull a rubber-like bouncy ball right out of the cup.
If you have trouble locating the ingredients, you can order them online here:
- Sodium Silicate (from Unit 3)
- Ethyl Alcohol (check your pharmacy)
- Disposable cups (at least two - and don't use your kitchen glassware, as you'll never get it clean again)
- Popsicle sticks (again, use something disposable to stir with)
Download Student Worksheet & Exercises
1. In one cup, measure four tablespoons of sodium silicate solution (it should be a liquid). Sodium silicate can be irritating to the skin for some people, so wear rubber gloves when doing this experiment!
2. Measure 1 tablespoon of ethyl alcohol into a second cup. Ethyl alcohol is extremely flammable—cap it and keep out of reach when not in use.
3. Pour the alcohol into the sodium silicate solution and stir with a Popsicle stick.
4. You’ll see a color change (clear to milky-white) and a state change (liquid to a solid clump.
5. Using gloves, gather up the polymer ball and firmly squeeze it in your hands.
6. Compress it into the shape you want—is it a sphere, or do you prefer a dodecahedron?
7. Bounce it!
8. Be patient when squeezing the compound together. If it breaks apart and crumbles, gather up the pieces and firmly press together.
Store your bouncy ball in a Ziploc bag!
What’s Going On?
Silicones are water repellent, so you’ll find that food dye doesn’t color your bouncy ball. You’ll find silicone in greases, oils, hydraulic fluids, and electrical insulators.
The sodium silicate is a long polymer chain of alternating silicon and oxygen atoms. When ethanol (ethyl alcohol) is added, it bridges and connects the polymer chains together by cross-linking them.
Think of a rope ladder—the wooden rungs are the cross-linking agents (the ethanol) and the two ropes are the polymer chains (sodium silicate).
Safety information for Sodium Silicate: MSDS.
Questions to Ask
1. Before the reaction, what was the sodium silicate like? Was it a solid, liquid, or gas? What color was it? Was it slippery, grainy, viscous, etc.?
2. What was the ethanol like before the reaction?
3. How is the product (the bouncy ball) different from the two chemicals in the beginning?
4. Was the bouncy ball the only molecule that was formed?
5. Was this reaction a physical or chemical change?
Did you know? Silly putty is actually a mixture of silicone and chalk!
When you think of slime, do you imagine slugs, snails, and puppy kisses? Or does the science fiction film The Blob come to mind? Any way you picture it, slime is definitely slippery, slithery, and just plain icky — and a perfect forum for learning real science.
But which ingredients work in making a truly slimy concoction, and why do they work? Let’s take a closer look…
Imagine a plate of spaghetti. The noodles slide around and don’t clump together, just like the long chains of molecules (called polymers) that make up slime. They slide around without getting tangled up. The pasta by itself (fresh from the boiling water) doesn’t hold together until you put the sauce on. Slime works the same way. Long, spaghetti-like chains of molecules don’t clump together until you add the sauce … until you add something to cross-link the molecule strands together.
The sodium-tetraborate-and-water mixture is the “spaghetti” (the long chain of molecules, also known as a polymer), and the “sauce” is the glue-water mixture (the cross-linking agent). You need both in order to create a slime worthy of Hollywood filmmakers.
Please login or register to read the rest of this content.
What state of matter is fire? Is it a liquid? I get that question a LOT, so let me clarify. The ancient scientists (Greek, Chinese… you name it) thought fire was a fundamental element. Earth, Air Water, and Fire (sometimes Space was added, and the Chinese actually omitted Air and substituted Wood and Metal instead) were thought to be the basic building blocks of everything, and named it an element. And it’s not a bad start, especially if you don’t have a microscope or access to the internet.
Today’s definition of an element comes from peeking inside the nucleus of an atom and counting up the protons. In a flame, there are lots of different molecules from NO, NO2, NO3, CO, CO2, O2, C… to name a few. So fire can’t be an element, because it’s made up of other elements. So, what is it?
Please login or register to read the rest of this content.
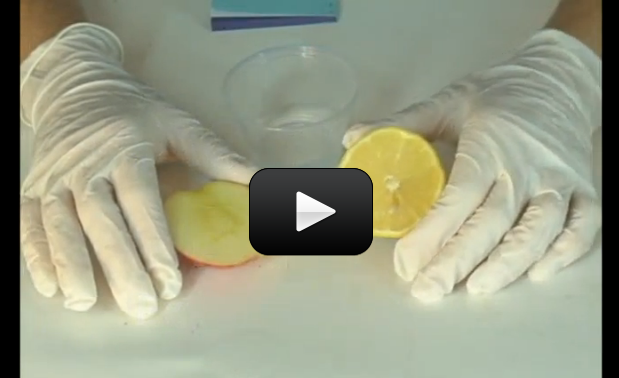
If you had a choice between a glass of lemon juice or apple juice, most folks would pick the sweeter one – apple. Did you know that apples are loaded with malic acid, and are actually considered to be acidic? It’s just that there is so much more sugar in an apple than a lemon that your taste buds can be fooled. Here’s a scientific way (which is much more reliable) to tell how acidic something is.
Acids are sour tasting (like a lemon), bases are bitter (like unsweetened cocoa powder). Substances in the middle are more neutral, like water. Scientists use the pH (power of hydrogen, or potential hydrogen) scale to measure how acidic or basic something is. Hydrochloric acid registers at a 1, sodium hydroxide (drain cleaner) is a 14. Water is about a 7. pH levels tell you how acidic or alkaline (basic) something is, like dirt. If your soil is too acidic, your plants won’t attract enough hydrogen, and too alkaline attracts too many hydrogen ions. The right balance is usually somewhere in the middle (called ‘pH neutral’). Some plants change color depending on the level of acidity in the soil – hydrangeas turn pink in acidic soil and blue in alkaline soil.
There are many different kinds of acids: citric acid (in a lemon), tartaric acid (in white wine), malic acid (in apples), acetic acid (in vinegar), and phosphoric acid (in cola drinks). The battery acid in your car is a particularly nasty acid called sulfuric acid that will eat through your skin and bones. Hydrochloric acid is found in your stomach to help digest food, and nitric acid is used to make dyes in fabrics as well as fertilizer compounds.
Please login or register to read the rest of this content.
This is the experiment that your audience will remember from your chemistry magic show. Here’s what happens – you call up six ‘helpers’ and hand each a seemingly empty test tube. Into each test tube, pour a little of the main gold-colored solution, say a few magic words, and their test tubes turn clear, black, pink, gold, yellow, and white. With a flourish, ask them to all pour their solutions back into yours and the final solution turns from inky black to clear. Voila!
I first saw a similar experiment when I was a kid, and I remembered it all the way through college, where I asked my professor how I could duplicate the experiment on my own. I was told that the chemicals used in that particular experiment were way too dangerous, and no substitute experiment was possible, especially for the color reversal at the end. I was determined to figure out an alternative. After two weeks of nothing but chemistry and experiment testing, I finally nailed it – and the best part is, you have most of these chemicals at the grocery store. (And the best part is, I can share it with you as I’ve eliminated the nasty chemicals so you don’t have to worry about losing an eyeball or a finger.)
NOTE: This experiment requires adult help, as it uses chemicals that are toxic if randomly mixed together. Follow the instructions carefully, and do not mix random chemicals together.
Are you ready to mix up your own rainbow?
Please login or register to read the rest of this content.
Hydrogen peroxide is used to fuel rockets, airplanes, and other vehicle engines. Chemistry teachers everywhere use it to demonstrate the power of a catalyst.
To speed up a reaction without altering the chemistry of the reaction involves adding a catalyst. A catalyst changes the rate of reaction but doesn’t get involved in the overall chemical changes.
For example, leaving a bottle of hydrogen peroxide outside in the sunlight will cause the hydrogen peroxide to decompose. However, this process takes a long time, and if you don’t want to wait, you can simply toss in a lump of charcoal to speed things along.
The carbon is a catalyst in the reaction, and the overall effect is that instead of taking two months to generate a balloon full of oxygen, it now only takes five minutes. The amount of charcoal you have at the end of the reaction is exactly the same as before it started.
A catalyst can also slow down a reaction. A catalytic promoter increases the activity, and a catalytic poison (also known as a negative catalyst, or inhibitor) decreases the activity of a reaction. Catalysts offer a different way for the reactants to become products, and sometimes this means the catalyst reacts during the chemical reaction to form intermediates. Since the catalyst is completely regenerated before the reaction is finished, it’s considered ‘not used’ in the overall reaction.
In this experiment, you'll see that there's a lot of oxygen hiding inside the peroxide - enough to really make things interesting and move around! You'll also find out what happens to soap when you bubble oxygen through it. Are you ready?
Please login or register to read the rest of this content.