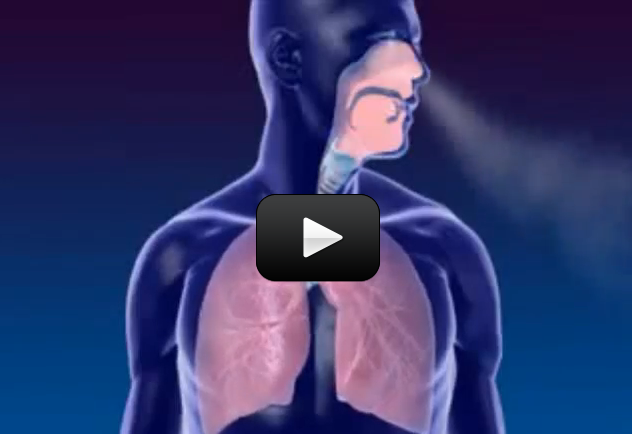
Take a deep breath in, and slowly let it out. As you do, think about the breaths you take without thinking about it. The truth is, you probably only think about breathing when you are coughing and having a hard time breathing. Even though breathing is not something we think about regularly, it is absolutely required for the survival of the cells in your body. As you breathe, oxygen flows into the body and carbon dioxide flows out. This very important exchange of gases is the main function of your body’s respiratory system.
Sometimes, people will say “breathing” and “respiration” as though they were the same thing, but they are actually very different. Breathing is the process where air enters the body, goes into the lungs, and exchanges its oxygen for carbon dioxide. This is part of respiration, and is known as external respiration, but it only half of full respiration. Respiration also includes internal respiration, where the blood, full of oxygen, goes to the parts of the body that need it. This process can be learned about in a discussion of the circulatory system, another body system.
 Although urine is sterile, it has hundreds of different kinds of wastes from the body. All sorts of things affect what is in your urine, including last night’s dinner, how much water you drink, what you do for exercise, and how well your kidneys work in the first place. This experiment will show you how the kidneys work to keep your body in top shape.
Although urine is sterile, it has hundreds of different kinds of wastes from the body. All sorts of things affect what is in your urine, including last night’s dinner, how much water you drink, what you do for exercise, and how well your kidneys work in the first place. This experiment will show you how the kidneys work to keep your body in top shape.