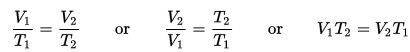This is a recording of a recent live teleclass I did with thousands of kids from all over the world. I’ve included it here so you can participate and learn, too!
You’ll discover how to boil water at room temperature, heat up ice to freeze it, make a fire water balloon, and build a real working steam boat as you learn about heat energy. You’ll also learn about thermal energy, heat capacity, and the laws of thermodynamics.
Materials:
- cup of ice water
- cup of room temperature water
- cup of hot water (not scalding or boiling!)
- tea light candle and lighter (with adult help)
- balloon (not inflated)
- syringe (without the needle)
- block of foam
- copper tubing (¼” diameter and 12” long)
- bathtub or sink
- scissors or razor
- fat marker (to be used to wrap things around, not for writing)