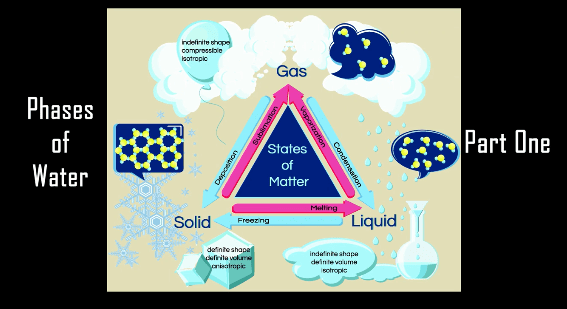
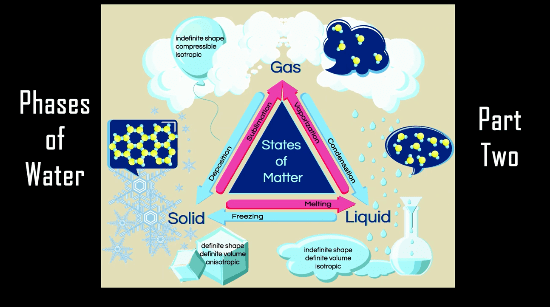
On March 14 at 1:59pm, folks from all over the world celebrate “Pi Day” with games, activities, and pie-eating contests. Here are my best resources for showing kids how pi shows up in the real world and also how to learn about pi in a way that not only makes sense but isn’t flat boring.
Pi (p) is a number slightly greater than 3 that shows up when you divide the circumference of a circle by its diameter, no matter what size the circle is. It also shows up in other shapes like spheres, ellipses, cylinders, and cones as well as unusual places like summation series, number theory, probability, bell curves, and the Fibonacci series.
I’ve prepared two different versions that you can access, and each comes with its own video. If this is your first time encountering “pi”, then start with the first one. Otherwise, jump in to the full version and have fun!
Please login or register to read the rest of this content.
Over the years, I’ve collected quite a stash of activity sheets and games for kids from other sources. I don’t know where they all came from, so please respect their copyright information on the sheet itself when you share with others.