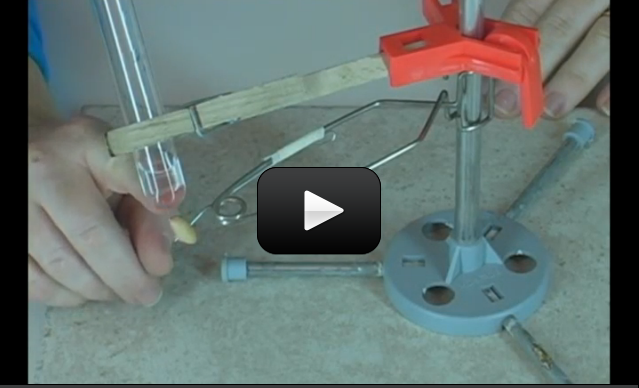
This experiment is for advanced students. Did you know that eating a single peanut will power your brain for 30 minutes? The energy in a peanut also produces a large amount of energy when burned in a flame, which can be used to boil water and measure energy.
Peanuts are part of the bean family, and actually grows underground (not from trees like almonds or walnuts). In addition to your lunchtime sandwich, peanuts are also used in woman’s cosmetics, certain plastics, paint dyes, and also when making nitroglycerin.
What makes up a peanut? Inside you’ll find a lot of fats (most of them unsaturated) and antioxidants (as much as found in berries). And more than half of all the peanuts Americans eat are produced in Alabama. We’re going to learn how to release the energy inside a peanut and how to measure it.
Please login or register to read the rest of this content.