
- Lesson 29: Fuel Cells
- Lesson 30: Fuel Cell Kit
- Lesson 31: Electric Vehicles
- Lesson 32: Electric Racecar
- Lesson 33: Magnifying Solar Power
- Lesson 34: Marshmallow Solar Roaster
- Lesson 35: Piezoelectric Effect
- Lesson 36: Stirling Engine
- Lesson 37: How to make a Stirling Engine from Soda Cans
- Lesson 38: Waste-to-Energy Facilities
- Lesson 39: Energy from Burning a Peanut
- Lesson 40: Finale
Quick Links:
Renewable & Alternative Energy 1
Renewable & Alternative Energy 2
[am4show have='p150;' guest_error='Guest error message' user_error='User error message' ]
Lesson 29: Fuel Cells
Lesson 30: Fuel Cell Kit
 This project is for advanced students. We’re going to build a car that runs entirely on sunlight and water. Use energy from the sun, we'll first use a solar cell to convert sunlight into electricity.
This project is for advanced students. We’re going to build a car that runs entirely on sunlight and water. Use energy from the sun, we'll first use a solar cell to convert sunlight into electricity.
Then we'll use that electricity to split the water molecule (H2O) into hydrogen and oxygen atoms and store them in separate tanks.
Lastly, we'll flip the system around to allow the hydrogen and oxygen gases to mix, which will produce the power to run the car and create an exhaust product that's just plain water.
You'll need to order the Fuel Cell Car Kit (Item# KT-FUELCCK from www.hometrainingtools.com). This kit is a bit expensive, but if you want to build a car that runs entirely from sunlight and water, this is the one you want to get. The company that makes this particular model also sells the conversion kits for (real!) cars.
Here's what you do:
How does that work? Molecules can also be split chemically, or by getting hit by a fast-moving particle. When you recombine the hydrogen and oxygen, energy is produced – enough to power a small car.
Back in 1800, William Nicholson and Johann Ritter were the first ones to split water into hydrogen and oxygen using electrolysis. (Soon afterward, Ritter went on to figure out electroplating.) They added energy in the form of an electric current into a cup of water and captured the bubbles forming into two separate cups, one for hydrogen and other for oxygen.
It takes energy to split a water molecule. (On the flip side, when you combine oxygen and hydrogen together, it makes water and a puff of energy. That’s what a fuel cell does.)
Back to splitting the water molecule – as the electricity zips through your wires, the water molecule breaks apart into smaller pieces: hydrogen ions (positively charged hydrogen) and oxygen ions (negatively charged oxygen). Remember that a battery has a plus and a minus charge to it, and that positive and negative attract each other.
So, the positive hydrogen ions zip over to the negative terminal and form tiny bubbles right on the wire. Same thing happens on the positive battery wire. After a bit of time, the ions form a larger gas bubble.
If you stick a cup over each wire, you can capture the bubbles and when you’re ready, ignite each to verify which is which.
Lesson 31: Electric Vehicles
Lesson 32: Electric Racecar
 Racerbots can steer because the two motors are activated independently. If you have more than one motor on your robot frame, you can turn either left, right, or spin on command. Wired remote control instructions follow this project.
Racerbots can steer because the two motors are activated independently. If you have more than one motor on your robot frame, you can turn either left, right, or spin on command. Wired remote control instructions follow this project.
These racerbots are the toughest of these robots to build. The wheels need to be squarely set on their shafts, all wheels need to be parallel, long wires out of the way, the motors spinning in the right direction, the battery pack in the right position... does this sound like a headache yet? Pay attention to construction details in the video and you’ll have less to fix later on.
Construction Tip: Cut the dowels in half to use for the axles and use the milk jug lids or film canister tops for wheels (you can also rip small, lightweight wheels off an old toy if they are about the size of a quarter). You may need to sand the dowels slightly if they are hard to fit into the wheels.
Materials:
- 4 popsicle sticks
- 1 straw
- 4 wheels or lids from film canisters, or milk jug lids (anything plastic, round, and about the size of a quarter)
- 1 skewer
- Two 3VDC motors
- AA battery case with AA batteries
- 2 alligator clip lead wires
- hot glue gun with glue sticks
Troubleshooting: To increase your motor speed, you’ll need to add a second battery pack. If you find the back wheels are slipping, run a bead of hot glue around the circumference of each wheel and carefully lay the flat side of a cut rubber band around the wheel (trim excess).
If the wheels spin in the opposite direction, your car will spin donuts (which could be fun!). If the car doesn’t move at all, use the basic circuit troubleshooting tips covered in previous experiments. (Are the batteries in the right way? Metal-to-metal connection? Fresh batteries? New wires?) Check to see if all four wheels spin freely without power. Roll the car down a ramp – does it travel relatively straight? If your robot keeps tripping over its wires, wrap the long lengths around the popsicle sticks or use shorter wires (cut in half, strip the insulation off, twist the exposed metal end on your electrical connections, and secure with tape).
What’s the next step? The instructions here are just for the chassis and propulsion systems of your robots. We’ll help you with the body framework and getting the robot to move. It’s up to you to add eyeballs, tentacles, claws, or whatever else you want to this framework.
Lesson 33: Magnifying Solar Power
Lesson 34: Marshmallow Solar Roaster
Do you like marshmallows cooked over a campfire? What if you don't have a campfire, though? We'll solve that problem by building our own food roaster - you can roast hot dogs, marshmallows, anything you want. And it's battery-free, as this device is powered by the sun.
NOTE: This roaster is powerful enough to start fires! Use with adult supervision and a fire extinguisher handy.
If you're roasting marshmallows, remember that they are white - the most reflective color you can get. If you coat your marshmallows with something darker (chocolate, perhaps?), your marshmallow will absorb the incoming light instead of reflecting it.
Here's what you need to get:
- 7x10” page magnifier (Fresnel lens)
- Cardboard box, about a 10” cube
- Aluminum foil
- Hot glue, razor, scissors, tape
- Wooden skewers (BBQ-style)
- Chocolate, marshmallows, & graham crackers
Here's what you do:
Download Student Worksheet & Exercises
How does it do that? The Fresnel lens is a lot like a magnifying glass. A convex lenses are thicker in the middle (you can feel it with your fingers). A Fresnel lens (first used in the 1800s to focus the beam in a lighthouse) has lots of ridges you can feel with your fingers. It's basically a series of magnifying lenses stacked together in rings (like in a tree trunk) to magnify an image.
The best thing about Fresnel lenses is that they are lightweight, so they can be very large (which is why light houses used these designs). Fresnel lenses curve to keep the focus at the same point, no matter close your light source is.
The Fresnel lens in this project is focusing the incoming sunlight much more powerfully than a regular hand held magnifier. But focusing the light is only part of the story with your roaster. The other part is how your food cooks as the light hits it. If your food is light-colored, it's going to cook slower than darker (or charred) food. Notice how the burnt spots on your food heat up more quickly!
Scientifically Dissecting a Marshmallow
Plants take in energy (from the sun), water, and carbon dioxide (which is carbon and oxygen) and create sugar, giving off the oxygen. In other words: carbon + water + energy = sugar
- In this experiment, we will reverse this equation, by roasting a marshmallow, which is mostly sugar.
- When you roast your marshmallow, first notice the black color. This is the carbon.
- Next notice the heat and light given off. These are two forms of energy.
- Finally, put the roasting marshmallow if a mason jar. Notice that condensation forms on the sides. This is the water.
So, by roasting the marshmallow, we showed: sugar = carbon + water + energy!
Exercises
Label the parts of the electromagnetic spectrum.
Lesson 35: Piezoelectric Effect
Lesson 36: Stirling Engine
Lesson 37: How to make a Stirling Engine from Soda Cans
This project is for advanced students.This Stirling Engine project is a very advanced project that requires skill, patience, and troubleshooting persistence in order to work right. Find yourself a seasoned Do-It-Yourself type of adult (someone who loves to fix things or tinker in the garage) before you start working on this project, or you'll go crazy with nit-picky things that will keep the engine from operating correctly. This makes an excellent project for a weekend.
Developed in 1810s, this engine was widely used because it was quiet and could use almost anything as a heat source. This kind of heat engine squishes and expands air to do mechanical work. There's a heat source (the candle) that adds energy to your system, and the result is your shaft spins (CD).
This engine converts the expansion and compression of gases into something that moves (the piston) and rotates (the crankshaft). Your car engine uses internal combustion to generate the expansion and compression cycles, whereas this heat engine has an external heat source.
This experiment is great for chemistry students learning about Charles's Law, which is also known as the Law of Volumes, which describes how gases tend to expand when they are heated and can be mathematically written like this:
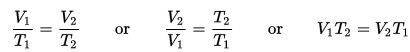
where V = volume, and T = temperature. So as temperature increases, volume also increases. In the experiment you're about to do, you will see how heating the air causes the diaphragm to expand which turns the crank.
Here's what you need:
- three soda cans
- old inner tube from a bike wheel
- super glue and instrant dry
- electrical wire (3- conductor solid wire)
- 3 old CDs
- one balloon
- penny
- nylon bushing (from hardware store)
- alcohol burner (you can build one out of soda cans or Sterno canned heat)
- fishing line (15lb. test or similar)
- pack of steel wool
- drill with 1/16" bit
- pliers
- scissors
- razor
- wire cutters
- electrical tape
- push pin
- permanent marker
- Swiss army knife (with can opener option)
- template
The Stirling heat engine is very different from the engine in your car. When Robert Stirling invented the first Stirling engine in 1816, he thought it would be much more efficient than a gasoline or diesel engine. However, these heat engines are used only where quiet engines are required, such as in submarines or in generators for sailboats.
Download Student Worksheet & Exercises
Here's how a Stirling engine is different from the internal-combustion engine inside your car. For example, the gases inside a Stirling engine never leave the engine because it's an external combustion engine. This heat engine does not have exhaust valves as there are no explosions taking place, which is why Stirling engines are quieter. They use heat sources that are outside the engine, which opens up a wide range of possibilities from candles to solar energy to gasoline to the heat from your hand.
There are lots of different styles of Stirling engines. In this project, we'll learn about the Stirling cycle and see how to build a simple heat engine out of soda cans. The main idea behind the Stirling engine is that a certain volume of gas remains inside the engine and gets heated and cooled, causing the crankshaft to turn. The gases never leave the container (remember - no exhaust valves!), so the gas is constantly changing temperature and pressure to do useful work. When the pressure increases, the temperature also increases. And when the temperature of the gases decreases, the pressure also goes down. (How pressure and temperature are linked together is called the "Ideal Gas Law".)
Some Stirling engines have two pistons where one is heated by an external heat source like a candle and the other is cooled by external cooling like ice. Other displacer-type Stirling engines has one piston and a displacer. The displacer controls when the gas is heated and cooled.
In order to work, the heat engine needs a temperature difference between the top and bottom of the cylinder. Some Stirling engines are so sensitive that you can simply use the temperature difference between the air around you and the heat from your hand. Our Stirling engine uses temperature difference between the heat from a candle and ice water.
The balloon at the top of the soda can is actually the 'power piston' and is sealed to the can. It bulges up as the gas expands. The displacer is the steel wool in the engine which controls the temperature of the air and allows air to move between the heated and cooled sections of the engine.
When the displacer is near the top of the cylinder, most of the gas inside the engine is heated by the heat source and gas expands (the pressure builds inside the engine, forcing the balloon piston up). When the displacer is near the bottom of the cylinder, most of the gas inside the engine cools and contracts. (the pressure decreases and the balloon piston is allowed to contract).
Since the heat engine only makes power during the first part of the cycle, there's only two ways to increase the power output: you can either increase the temperature of the gas (by using a hotter heat source), or by cooling the gases further by removing more heat (using something colder than ice).
Since the heat source is outside the cylinder, there's a delay for the engine to respond to an increase or decrease in the heat or cooling source. If you use only water to cool your heat engine and suddenly pop an ice cube in the water, you'll notice that it takes five to fifteen seconds to increase speed. The reason is because it takes time for the additional heat (or removal of heat by cooling) to make it through the cylinder walls and into the gas inside the engine. So Stirling engines can't change the power output quickly. This would be a problem when getting on the freeway!
In recent years, scientists have looked to this engine again as a possibility, as gas and oil prices rise, and exhaust and pollutants are a concern for the environment. Since you can use nearly any heat source, it's easy to pick one that has a low-fume output to power this engine. Scientists and engineers are working on a model that uses a Stirling engine in conjunction with an internal-combustion engine in a hybrid vehicle... maybe we'll see these on the road someday!
Exercises
- What is the primary input of energy for the Stirling engine?
- As Pressure increases in a gas, what happens to temperature?
- It increases
- Nothing
- It decreases
- It increases, then decreases
- What is the primary output of the Stirling engine?
Lesson 38: Waste-to-Energy Facilities
Lesson 39: Energy from Burning a Peanut
Did you know that eating a single peanut will power your brain for 30 minutes? The energy in a peanut also produces a large amount of energy when burned in a flame, which can be used to boil water and measure energy.
Peanuts are part of the bean family, and actually grows underground (not from trees like almonds or walnuts). In addition to your lunchtime sandwich, peanuts are also used in woman's cosmetics, certain plastics, paint dyes, and also when making nitroglycerin.
What makes up a peanut? Inside you'll find a lot of fats (most of them unsaturated) and antioxidants (as much as found in berries). And more than half of all the peanuts Americans eat are produced in Alabama. We're going to learn how to release the energy inside a peanut and how to measure it.
Materials:
- raw peanuts
- chemistry stand with glass test tube and holder (watch video)
- flameproof surface (large ceramic tile or cookie sheet)
- paper clip
- alcohol burner or candle with adult help
- fire extinguisher
Download Student Worksheet & Exercises
What's Going On? There's chemical energy stored inside a peanut, which gets transformed into heat energy when you ignite it. This heat flows to raise the water temperature, which you can measure with a thermometer. You should find that your peanut contains 1500-2100 calories of energy! Now don't panic... this isn't the same as the number of calories you're allowed to eat in a day. The average person aims to eat around 2,000 Calories (with a capital "C"). 1 Calorie = 1,000 calories. So each peanut contains 1.5-2.1 Calories of energy (the kind you eat in a day). Do you see the difference?
But wait... did all the energy from the peanut go straight to the water, or did it leak somewhere else, too? The heat actually warmed up the nearby air, too, but we weren't able to measure that. If you were a food scientist, you'd use a nifty little device known as a bomb calorimeter to measure calorie content. It's basically a well-insulated, well-sealed device that catches nearly all the energy and flows it to the water, so you get a much more accurate temperature reading. (Using a bomb calorimeter, you'd get 6.1-6.8 Calories of energy from one peanut!)
How do you calculate the calories from a peanut?
Let's take an example measurement. Suppose you measured a temperature increase from 20 °C to 100 °C for 10 grams of water, and boiled off 2 grams. We need to break this problem down into two parts - the first part deals with the temperature increase, and the second deals with the water escaping as vapor.
The first basic heat equation is this:
Q = m c T
Q is the heat flow (in calories)
m is the mass of the water (in grams)
c is the specific heat of water (which is 1 degree per calorie per gram)
and T is the temperature change (in degrees)
So our equation becomes: Q = 10 * 1 * 80 = 800 calories.
If you measured that we boiled off 2 grams of water, your equation would look like this for heat energy:
Q = L m
L is the latent heat of vaporization of water (L= 540 calories per gram)
m is the mass of the water (in grams)
So our equation becomes: Q = 540 * 2 = 1080 calories.
The total energy needed is the sum of these two:
Q = 800 calories + 1080 calories = 1880 calories.
Lesson 40: Finale
[/am4show]
