
NOTE: If you haven't already, please log in using the form on the right of your screen. If there's no login box, then you're already logged in!
Discover the world of clean, renewable energy that scientists are developing today! Explore how they are harnessing the energy of tides and waves, lean how cars can run on just sunlight and water, tour a hydroelectric power plant, visit the largest wind farms on the planet, and more!
You’ll learn how streets are being designed to generate electricity, how teenagers are making jet fuel from pond scum in their garage, and how 70 million tons of salt can provide free, clean energy 24 hours a day forever! During class, you’ll learn how to bake solar cookies, magni-fry marshmallows and do the experiment with light Einstein won a Nobel prize for that is the basis of all photovoltaic energy today.
Renewable energy is energy that is generated from natural processes that are continuously replenished. This includes sunlight, geothermal heat, wind, tides, water, and various forms of biomass. This energy cannot be exhausted and is constantly renewed.
Alternative energy is a term used for an energy source that is an alternative to using fossil fuels. Generally, it indicates energies that are non-traditional and have low environmental impact. The term alternative is used to contrast with fossil fuels according to some sources. By most definitions alternative energy doesn't harm the environment, a distinction which separates it from renewable energy which may or may not have significant environmental impact.
[am4show have='p148;' guest_error='Guest error message' user_error='User error message' ]
 We’re going to cover the following scientific concepts:
We’re going to cover the following scientific concepts:
|
|
Step 1:
We’re about to dive into a comprehensive course that teaches the big ideas that are currently being developed by engineers and scientists in the renewable energy and alternate energy field. Soon you’ll learn how to build steam engines, understand how generators work, build a photoelectric experiment, and construct a vehicle that runs on sunlight and water by studying energy, magnetism, electricity, and so much more!
To get started learning, print out this workbook, which includes a complete shopping list inside. You'll be filling out this workbook out as you work through the course. The material list inside the workbook are materials for the experiments covered in the course below. You don't need to do all the experiments to get a great education in Renewable & Alternative Energy! Simply pick and choose the projects and experiments you want to do based on time, budget and interest, and work from there!
Step 2:
Did you print out the workbook yet? Make sure you have it in front of you before you watch the videos below! If you have the materials to do the experiments, feel free to do the experiments along with me. If not, just watch the videos so you still get the experience of what the experiment is all about. Some of the experiments have additional worksheets you can print out and fill in as you work through the experiment.
- Lesson 1: Introduction
- Lesson 2:Atmosphere
- Lesson 3: Burning Coal
- Lesson 4: Nuclear Energy
- Lesson 5: Solar Cells & Photons
- Lesson 6: Photoelectric Effect
- Lesson 7: Solar cells
- Lesson 8: Solar Boat
- Lesson 9: Solar Car
- Lesson 10: Solar Thermal Power
- Lesson 11: Solar Cookies
- Lesson 12: Solar Battery
Quick Links:
Renewable & Alternative Energy 2
Renewable & Alternative Energy 3
Lesson 1: Introduction
Lesson 2: Atmoshere
Lesson 3: Burning Coal
Bituminous coal (also called black coal) is a soft, black organic sedimentary rock that contains 85% carbon. It’s a lower grade than anthracite coal, which contains 93% carbon. Bituminous coal can either be dull or shiny, whereas anthracite is hard and shiny. Lignite, a lower grade than bituminous, is a crumbly, black type of coal that only contains 72% carbon.
Materials
- Votive candle
- Paperclip
- Hammer (if your piece of coal is large)
- Pliers (to bend paperclip)
- Lighter with adult help
- Cup of water
- Rock samples (in the video: bituminous coal, anthracite coal)
Download worksheet and exercises
Coal comes in two main forms: bituminous and anthracite. Bituminous coal is the stuff used to generate energy, whether electricity, heated water, or as a product in other substances. It’s more abundant than anthracite, which is the purer version of coal. Anthracite burns hotter and cleaner, but it’s more scarce. Coal also exists in two other forms as peat, which used to serve as fuel for heating homes as well as lignite, which is more pure than peat but not quite as pure as bituminous.
Coal is one of the two kinds of rocks that are not made from minerals (amber is the other).
Today’s lab lets you burn coal to identify the byproducts of combustion. When coal is burned, it releases volatile compounds that are hazardous to your health, including methane, hydrogen, carbon monoxide, carbon dioxide, nitrogen, and volatile hydrocarbons, but the chemical reaction leaves behind a purer form of carbon than found in the coal itself. So make sure to do this experiment outside!
- Move your experiment outside. Do not do this indoors! Fumes generated must be properly ventilated.
- Open up and bend the paperclip into a shape that will allow you to hold the coal without it falling off the end. Use pliers to bend the paperclip.
- Set a small piece (about the size of a pea) of bituminous coal at the end of the paperclip.
- Put on your goggles. NO EXCEPTIONS!
- Have an adult light your candle.
- Place the coal piece at the top of the flame for the most intense heat.
- Look for brown to black smoke to come from the coal. Record your observations in your data table.
- When you’re done, place the entire piece of coal in your cup of water.
Optional: If you’ve got a test tube and stand, you can do the following experiment:
- Put on your goggles, and pick up the test tube with the test tube holder.
- Place a small piece of bituminous coal in the test tube (about the size of a pea).
- Place the tube at an angle in a proper holder.
- Turn on the heat source, and wave it near the bottom of the test tube for a few minutes, until you notice any slight changes.
- As your coal sample heats up, look for bubbles or gas, which is coal gas, one of the first of three things produced by coal. The coal gas was captured and used as fuel in household lamps and streetlights before electricity was available.
- As you continue to heat the sample, look for brown stuff along the sides of the tube called coal tar, which is used in dyes, aspirin, textiles, pesticides, and more.
- As your sample continues to burn, notice if there are any gray-black solids in the bottom. This will look a lot like ash, but it’s called coke and it’s used to purify metals in a chemical process.
Exercises
- What are the three products that coal generates?
- Name four types of coal.
- What are two things we use coal for?
- What type of coal is the most pure?
- What is the dominant element in coal?
- What are three alternatives to generating energy, instead of using coal?
Lesson 4: Nuclear Energy
Lesson 5: Solar Cells & Photons
Lesson 6: Photoelectric Effect
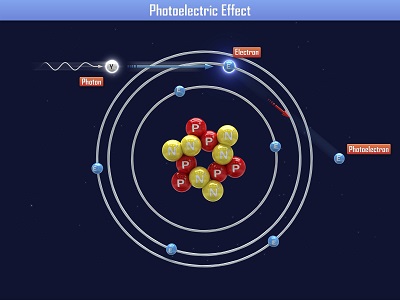 Einstein received a Nobel Prize for figuring out what happens when you shine blue light on a sheet of metal. When he aimed a blue light on a metal plate, electrons shot off the surface. (Metals have electrons which are free to move around, which is why metals are electrically conductive. More on this in Unit 10).
Einstein received a Nobel Prize for figuring out what happens when you shine blue light on a sheet of metal. When he aimed a blue light on a metal plate, electrons shot off the surface. (Metals have electrons which are free to move around, which is why metals are electrically conductive. More on this in Unit 10).
When Einstein aimed a red light at the metal sheet, nothing happened. Even when he cranked the intensity (brightness) of the red light, still nothing happened. So it was the energy of the light (wavelength), not the number of photons (intensity) that made the electrons eject from the plate. This is called the ‘photoelectric effect’. Can you imagine what happens if we aim a UV light (which has even more energy than blue light) at the plate?
This photoelectric effect is used by all sorts of things today, including solar cells, electronic components, older types of television screens, video camera detectors, and night-vision goggles.
This photoelectric effect also causes the outer shell of orbiting spacecraft to develop an electric charge, which can wreck havoc on its internal computer systems.
A surprising find was back in the 1960s, when scientists discovered that moon dust levitated through the photoelectric effect. Sunlight hit the lunar dust, which became (slightly) electrically charged, and the dust would then lift up off the surface in thin, thread-like fountains of particles up ¾ of a mile high.
Materials:
- soda or steel can
- paper clip
- sand paper
- tinsel (or aluminum foil and scissors)
- tape
- foam cup
- PVC pipe (any size)
- brown paper bag
- UV shortwave lamp (sometimes called a "germ-free portable lamp")
Lesson 7: Solar cells
Lesson 8: Solar Boat
 Does it really matter what angle the solar cell makes with the incoming sunlight? If so, does it matter much? When the sun moves across the sky, solar cells on a house receive different amounts of sunlight. You’re going to find out exactly how much this varies by building your own solar boat.
Does it really matter what angle the solar cell makes with the incoming sunlight? If so, does it matter much? When the sun moves across the sky, solar cells on a house receive different amounts of sunlight. You’re going to find out exactly how much this varies by building your own solar boat.
- Solar motor
- Solar cell
- Foam block (about 6” long)
- Alligator clip leads
- Propeller (you can rip one off an old small personal fan or old toy, or find them at hobby stores)
Here's what you do:
Download Student Worksheet & Exercises
1. Attach the wires of the solar cell to the motor (one to each motor terminal).
2. Attach the propeller to your motor. If the shaft won’t fit, drill out the center hole. If the hole is too large, use a tiny dab of hot glue on the shaft tip to secure the propeller into place.
3. Stand out in the sun. How do you need to hold your solar cell to make the propellers pin the fastest?
4. Position the motor on a block of foam so that the propeller hangs off the edge and is free to rotate. Hot glue the motor into place, being careful not to get any hot glue near any vents in your motor.
5. Hot glue your solar cell to the foam block. You might want to check the final position in sunlight before attaching it.
6. Optional: Wire up a simple switch (from Unit 10) using paperclips and brass fasteners so you can easily turn your power on and off.
Exercises Answer the questions below:
- What kind of electricity comes from a battery and photovoltaic cell?
- Nuclear
- Voltaic
- Electrochemical
- Ionized
- Electricity is another name for the free flow of:
- Protons
- Quarks
- Electrodes
- Electrons
- True or false: Ions are attracted to the same charge.
- True
- False
- Do solar panels work in cloudy climates?
- Yes
- No
Lesson 9: Solar Car
 Solar energy (power) refers to collecting this energy and storing it for another use, like driving a car. The sun blasts 174 x 1015 watts (which is 174,000,000,000,000,000 watts) of energy through radiation to the earth, but only 70% of that amount actually makes it to the surface. And since the surface of the earth is mostly water, both in ocean and cloud form, only a small fraction of the total amount makes it to land.
Solar energy (power) refers to collecting this energy and storing it for another use, like driving a car. The sun blasts 174 x 1015 watts (which is 174,000,000,000,000,000 watts) of energy through radiation to the earth, but only 70% of that amount actually makes it to the surface. And since the surface of the earth is mostly water, both in ocean and cloud form, only a small fraction of the total amount makes it to land.
A solar cell converts sunlight straight into electricity. Most satellites are powered by large solar panel arrays in space, as sunlight is cheap and readily available out there. While solar cells seem 'new' and modern today, the first ones were created in the 1880s, but were a mere 1% efficient. (Today, they get as high as 35%.) A solar cell's efficiency is a measure of how much sunlight the cell converts into electrical energy.
- Solar cell
- Solar motor
- Foam block (about 6” long)
- Alligator clip leads
- 2 straws (optional)
- 2 wooden skewers (optional)
- 4 milk jug lids or film can tops
- Set of gears, one of which fits onto your motor shaft (most solar motor kits come with a set), or rip a set out of an old toy
Here's what you do:
Download Student Worksheet & Exercises
How does a solar cell work? Solar cells are usually made of silicon. Sunlight is made of packets of energy called photons. When photons hit the silicon, one of three things can happen: the photons can pass straight through the silicon if they have a low enough energy; they can get reflected off the surface; or (and this is the fun part) they get absorbed and the electrons in the silicon get knocked out of their shell.
Once knocked out of orbit, the free electrons start flowing through the silicon to create electricity. The solar cells are structured is such a way as to keep the electricity flowing only in one direction. The electron flow created is DC current (refer to Unit 10).
The solar cells you can buy from stores require huge amounts of energy in creating the solar cell, which is the primary downside. You need high temperatures, big vacuum pumps, and lots of people to make a set of solar cells. However, if we focus just on the physics of the solar cell, then we can easily create our own solar battery and other solar cell projects using household items. While these cells won't look as spiffy as the ones from the store, they still produce electricity from sunlight.
Exercises Answer the questions below:
- Most solar cells are made of what material?
- Hydrogen
- Aluminum
- Silicon
- Titanium
- Name one benefit of solar cells and one drawback of using them for electricity.
- Benefit:
- Drawback:
- Electrical current begins flowing when:
- Sunlight hits an atom
- Electrons are knocked out of orbiting atoms
- Protons get charged
- An atom’s nucleus splits
Lesson 10: Solar Thermal Power
Lesson 11: Solar Cookies
Can you use the power of the sun without using solar cells? You bet! We're going to focus the incoming light down into a heat-absorbing box that will actually cook your food for you.
Remember how we learned about photons (packets of light)? Sunlight at the Earth's surface is mostly in the visible and near-infrared (IR) part of the spectrum, with a small part in the near-ultraviolet (UV). The UV light has more energy than the IR, although it's the IR that you feel as heat.
We're going to use both to bake cookies in our homemade solar oven. There are two different designs - one uses a pizza box and the other is more like a light funnel. Which one works best for you?
- Two large sheets of poster board (black is best)
- Aluminum foil
- Plastic wrap
- Black construction paper
- Cardboard box
- Pizza box (clean!)
- Tape & scissors
- Reusable plastic baggies
- Cookie dough (your favorite)
Download Student Worksheet & Exercises
How does that work? Your solar cooker does a few different things. First, it concentrates the sunlight into a smaller space using aluminum foil. This makes the energy from the sun more potent. If you used mirrors, it would work even better!
You're also converting light into heat by using the black construction paper. If you've ever gotten into car with dark seats, you know that those seats can get HOT on summer days! The black color absorbs most of the sunlight and transforms it into heat (which boosts the efficiency of your solar oven).
By strapping on a plastic sheet over the top of the pizza-box cooker, you're preventing the heat from escaping and cooling the oven off. Keeping the cover clear allows sunlight to enter and the heat to stay in. (Remember the black stuff converted your light into heat?) If you live in an area that's cold or windy, you'll find this part essential to cooking with your oven!
Here's another type of solar cooker that uses a cone design to focus the energy straight to your cookies!
Exercises Answer the questions below:
- Name the type of heat energy that the sun provides:
- Convection
- Conduction
- Radiation
- Invection
- What are some ways that the sun’s energy can be directly harnessed?
- Name three of the different parts of the electromagnetic spectrum:
Lesson 12: Solar Battery
This is the kind of energy most people think of when you mention 'alternative energy', and for good reason! Without the sun, none of anything you see around you could be here. Plants have known forever how to take the energy and turn it into usable stuff… so why can't we?
The truth is that we can. While normally it takes factories the size of a city block to make a silicon solar cell, we'll be making a copper solar cell after a quick trip to the hardware store. We're going to modify the copper into a form that will allow it to react with sunlight the same way silicon does. The image shown here is the type of copper we're going to make on the stovetop.
This solar cell is a real battery, and you'll find that even in a dark room, you'll be able to measure a tiny amount of current. However, even in bright sunlight, you'd need 80 million of these to light a regular incandescent bulb.
You'll need to gather these materials together:
- ½ sq. foot of copper flashing sheet (check the scrap bin at a hardware store)
- Alligator clip leads
- Multimeter
- Electric stove (not gas)
- Large plastic 2L soda bottle
- ¼ cup salt
- Sandpaper & sheet metal shears
Here's what you need to do:
Download Student Worksheet & Exercises
How does that work? Do you remember learning about the photoelectric effect? This cuprous oxide solar cell ejects electrons when placed in UV light - and sunlight has enough UV light to make this solar cell work. Those free electrons are now free to flow - which is exactly what we're measuring with the volt meter.
Semiconductors are the secret to making solar cells. A semiconductor is a material that is part conductor, part insulator, meaning that electricity can flow freely and not, depending on how you structure it. There are lots of different kinds of semiconductors, including copper and silicon.
In semiconductors, there's a gap (called the bandgap) that's like a giant chasm between the free electrons (electrons knocked out of its shell) and bound electrons (electrons attached to an atom). Electrons can be either free or attached, but it costs a certain amount of energy to go either way (like a toll both).
When sunlight hits the semiconductor material in the solar cell, some of the electrons get enough energy to jump the gap and get knocked out of their shell to become free electrons. The free electrons zip through the material and create a low of electrons. When the sun goes down, there's no source of energy for electrons to get knocked out of orbit, so they stay put until sunrise.
Does it really matter what angle the solar cell makes with the incoming sunlight? If so, does it matter much? When the sun moves across the sky, solar cells on a house receive different amounts of sunlight.
Exercises Answer the questions below:
- The sunlight causes the electrons to flow from the cuprous oxide because of:
- Photosynthesis
- The electromagnetic spectrum
- The photoelectric effect
- The photochemical principle
- What material do most solar cells use instead of copper?
- What part of the electromagnetic spectrum is most active in this experiment?
- Visible Light
- Ultraviolet Light
- Gamma Rays
- Microwaves
- When you read amps, you read:
- Current
- Voltage
- Power Draw
- Work
Click Here to go to Next Lesson !
[/am4show]
