 If you think about it, the nucleus of an atom (proton and neutron) really have no reason to stick together. The neutron doesn’t have a charge, and the proton has a positive charge. And most nuclei have more than one proton, and positive-positive charges repel (think of trying to force two North sides of a magnet together). So what keeps the core together?
If you think about it, the nucleus of an atom (proton and neutron) really have no reason to stick together. The neutron doesn’t have a charge, and the proton has a positive charge. And most nuclei have more than one proton, and positive-positive charges repel (think of trying to force two North sides of a magnet together). So what keeps the core together?
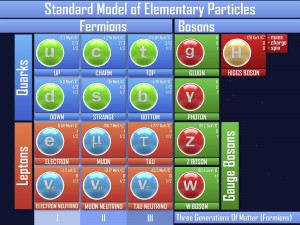
The strong force. Well, actually the residual strong force. This force is the glue that sticks the nucleus of an atom together, and is one of the strongest force we’ve found (on its own scale). This force binds the protons and neutrons together and is carried by tiny particles called pions. When you split apart these bonds, the energy has to go somewhere… which is why fission is such a powerful process (more on that later).
The fundamental strong force holds the quarks together inside the proton and neutron. Itty bitty particles called gluons hold the quarks together so the atom doesn’t fly apart. This force is extremely strong – much stronger than the electromagnetic force. This force is also known as the color force (there is not any color involved – that is just the way it was named.)
The electromagnetic force keeps the electrons from flying away from the nucleus. When a plus (the nucleus) and minus (the electron) charge get close together, tiny particles called photons pull the two together.
What is Radiation?
Radiation is energy or parts of atoms that are given off. We measure radiation with a geiger counter, which has a tube of gas inside that every time it gets hit by radiation, it gives off a little electrical charge.


I have updated the information and you’ll find everything you need here:
https://www.sciencelearningspace2.com/2014/09/science-teleclass-astronomy/
There are three main sources of radiation: cosmic (from outer space), terrestrial (from the earth’s crust), and internal (from the human body). There are many natural sources of radiation, and in the last 100 years, we’ve also added artificial radiation sources. The naturally occurring sources make up 5X as much as the artificial ones.
Radiation is the natural process of high-energy particles traveling through space (radiating from a source). Alpha particles (α) are stopped by a sheet of paper while beta particles (β) are stopped by an aluminium plate. Gamma radiation (γ) is dampened when it penetrates matter, and these are the most harmful of the three types.
So is it correct to say that there is more radiation in our world now than in the past? Or is radiation “matter” which cannot be destroyed or created?
Slideshow:
Astrophysics/Getting started/lesson 2
The one on stars and planets…
You need special equipment to detect gamma rays, since you can’t see or feel the radiation hitting your body. Your exposure to x-rays is almost entirely from dental and medical x-rays. You cal always ask the lab tech or doctor about the necessity of having an x-ray, and ask how recently the x-ray machine has been inspected and properly calibrated. It is possible that you may encounter an instrument or device containing a gamma radiation source. Factories that have gone out of business may contain radioactive devices, and are unknowingly dismantled and sold as scrap metal. These devices often bear the radiation symbol, which means the device is radioactive. Our upper atmosphere absorbs most of the x-rays and gamma rays from space.
Which slideshow? Can you tell me the page you are looking at?
X-rays, gamma rays, and charged particles are types of radiation used for cancer treatment. Here’s an article you might find useful.
What is used for cancer radiation treatment? Is it still radium?
Could you please tell me how to turn the pics for the ‘Getting Started’ slideshow? Thanks.
Where are gamma rays found?