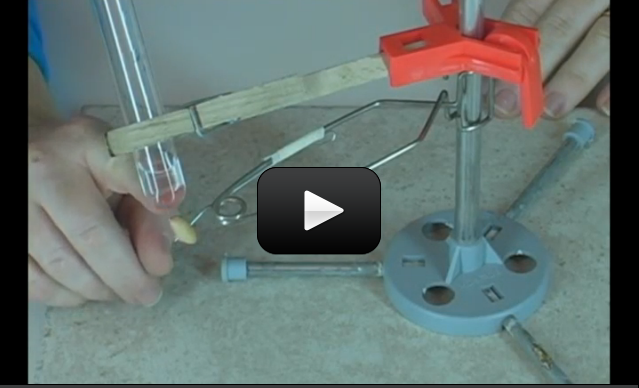
This experiment is for advanced students. Did you know that eating a single peanut will power your brain for 30 minutes? The energy in a peanut also produces a large amount of energy when burned in a flame, which can be used to boil water and measure energy.
Peanuts are part of the bean family, and actually grows underground (not from trees like almonds or walnuts). In addition to your lunchtime sandwich, peanuts are also used in woman’s cosmetics, certain plastics, paint dyes, and also when making nitroglycerin.
What makes up a peanut? Inside you’ll find a lot of fats (most of them unsaturated) and antioxidants (as much as found in berries). And more than half of all the peanuts Americans eat are produced in Alabama. We’re going to learn how to release the energy inside a peanut and how to measure it.
Please login or register to read the rest of this content.

This one is tricky because air heats up fast, so I would recommend adding a little bit (no more than 2 tablespoons) of water to the bottom of the container and then freeze it overnight. See if that helps, and let me know what happens!
Miss Aurora,
I did the project called “Ghost Coin” and the coin did not jump. Do you think it has something to do with the type of plastic bottle? I left it in the freezer at least overnight. Can it be left in the freezer for too long? When I took the bottle out of the freezer it took me several seconds to get the coin on the top. Maybe the bottle didn’t stay cold enough? The coin also was a quarter and was a little too big but the other coins I tried were too small. Does the coin size matter as long as it fits? Thank you.
-J
I was waiting for the wooden clip to catch fire too! 😀
Hmm… that’s a really good question. I haven’t tried it with other nuts, but I wonder what would happen if you try a skinned almond (you can blanch it to get the skins off), or a walnut, or…? Give it a try and let me know! That’s exactly the kind of thing a real scientist would do… ask questions, and then set up an experiment to test it out! 🙂 Let me know how it goes.
What if we have kids who are allergic to peanuts? Could you suggest changes for using something else comparable? i know that peanuts are not nuts, but nuts do have a lot of calories, as well. Would a pecan or walnut or cashew work?
Thank you!
Hi, where can I get the chemistry stand with glass test tube and holder? Thanks