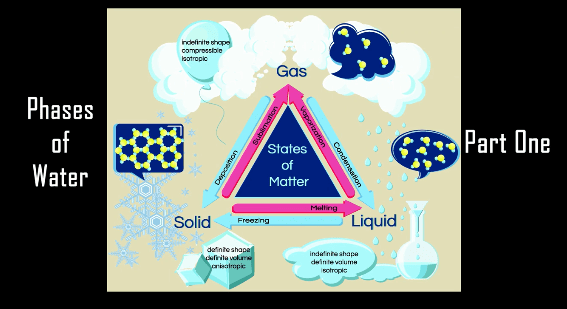
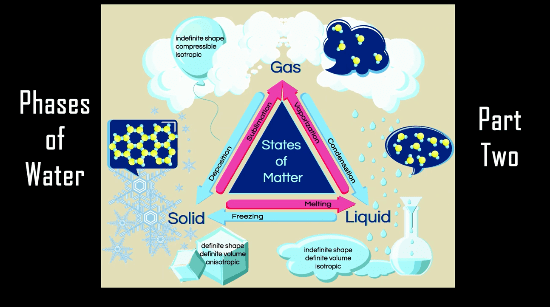
Today we will investigate the reducing power of orange.
Please login or register to read the rest of this content.
This experiment is for advanced students.
Sparks flying off in all directions…that’s fun. In this lab, we will show how easy it is to produce those shooting sparks. In a sparkler you buy at the store, the filings used are either iron or aluminum.
The filings are placed in a mixture that, when dry, adheres to the metal rod or stick that is used in making the sparkler. The different colors are created by adding different powdered chemicals to the mixture before it dries. When they burn, we get red, blue, white, and green.
Please login or register to read the rest of this content.
Click here to go to next lesson on Limiting Reactants.
A lot of chemical reactions happen in a solution (it allows the chemicals to interact much more easily with each other when it is), so chemists define how much of the solute is in the solution by the term MOLARITY.
Molarity is a really convenient unit of concentration and it works like this. If I have 10 moles of solute in 10 liters of water, what’s the molarity? 10/10 = 1! So it’s a 1M solution. What if I have 20 moles in 10 liters? Then it’s a 2M solution. See how easy that is?
Please login or register to read the rest of this content.
Click here to go to next lesson on Iron Sparklers.
Precipitate reactions are like watching a snow globe, but the snow appears out of nowhere.
For example, you can combine two liquid solutions that are totally clear and when you put them together, they each break apart into ions and then recombine in a way that looks like white snow in your test tube. Basically precipitate reactions make it possible to see the ions in a solution because they form a salt that’s not soluble – it doesn‘t dissolve in the solution. You can also get different colors of the precipitate snow, depending on which reactants you start out with. If you were to use potassium bromide (KBr) with silver nitrate, you’d find a yellowish snowstorm of silver bromide (AgBr).
Please login or register to read the rest of this content.
Click here to go to next lesson on Electrolytes.
We’re going to be mixing up dinosaur toothpaste, doing experiments with catalysts, discovering the 5 states of matter, and building your own chemistry lab station as we cover chemical kinetics, phase shifts, the states of matter, atoms, molecules, elements, chemical reactions, and much more. We’re also going to turn liquid polymers into glowing putty so you can amaze your friends when it totally glows in the dark. AND make liquids freeze by heating them up (no kidding) using a scientific principle called supercooling,
Materials:
- Chemistry Worksheet
- Aluminum pie plate
- Bowl
- Clear glue or white glue
- Disposable cups
- Goggles & gloves
- Hydrogen peroxide
- OPTIONAL: Instant reusable hand warmer (containing sodium acetate )
- Liquid soap
- Popsicle sticks
- Scissors or pliers
- Sodium tetraborate (also called “Borax”)
- Water bottle
- Yeast
- Yellow highlighter
- Optional: If you want to see your experiments glow in the dark, you'll need a fluorescent UV black light (about $10 from the pet store - look in cleaning supplies under "Urine-Off" for a fluorescent UV light). UV flashlights and UV LEDs will not work.
This experiment is for advanced students. Hydrolysis is a chemical reaction that involves breaking a molecular bond using water. In chemistry, there are three different types of hydrolysis: sat hydrolysis, acid hydrolysis, and base hydrolysis. In nature, living organisms survive by making their energy from processing food. The energy converted from food is stored in ATP molecules. To release the energy stored in food, a phosphate group breaks off an ATP molecule (and becomes ADP) using hydrolysis and releases energy from the bonds.
Please login or register to read the rest of this content.